Label: JEANATOPE- iodinated i-125 albumin injection, solution
- NDC Code(s): 50914-7733-5
- Packager: Iso-Tex Diagnostics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Chemical Characteristics
JEANATOPE (iodinated I 125 albumin) injection is a sterile, nonpyrogenic radioactive diagnostic agent for intravenous use. Each mL contains albumin human (approximately 10 mg), dibasic sodium phosphate (16 mg), guanidine hydrochloride (not more than 0.4 mg), monobasic sodium phosphate (1.6 mg), sodium chloride for isotonicity, and benzyl alcohol (9 mg) as a preservative. The stabilizers acetyltryptophanate and sodium caprylate have a concentration of less than 0.89 mM. The pH has been adjusted to 7.2 to 7.8 with sodium hydroxide or hydrochloric acid. Each vial contains 37 MBq/mL (1,000 microCi/mL) of radioactivity as iodinated I 125 albumin at time of calibration (see HOW SUPPLIED).Physical Characteristics
Iodine-125 decays by electron capture with a physical half-life of 60.14 days. Photons that are useful for detection and imaging studies are listed in Table 1.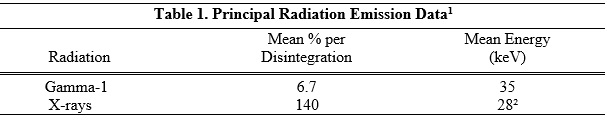
1Dillman LT, Von der Lage FC; Radionuclide Decay Schemes and Nuclear Parameters for Use in Radiation-Dose Estimation. MIRD Pamphlet No. 10, pg.71 Soc Nucl Med, 1975.
²Weighted mean energyExternal Radiation
The specific gamma ray constant for iodine-125 is 1.5 R/millicurie-hour at 1 cm. The first half-value thickness of Pb for iodine-125 is 0.02 mm. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of Pb is shown in Table 2. For example, the use of 0.2 mm of Pb will decrease the external radiation exposure by a factor of 1,000.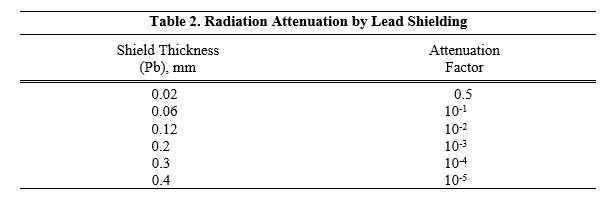
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals before and after the time of calibration are shown in Table 3.
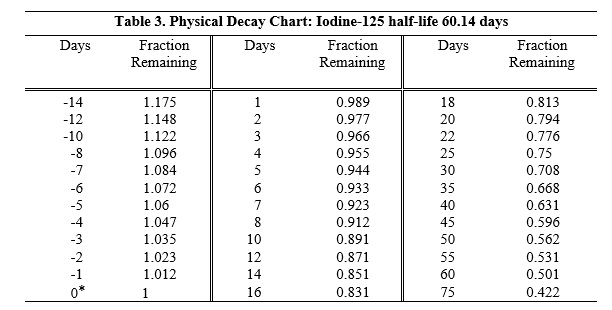
*Calibration time
-
CLINICAL PHARMACOLOGY
Following intravenous injection, iodinated I 125 albumin is distributed throughout the intravascular pool within 10 minutes; extravascular distribution takes place more slowly. Iodinated I 125 albumin also can be detected in the lymph and in certain body tissues within 10 minutes after injection, but maximum distribution of radioactivity throughout the extravascular space does not occur until two to four days after administration. The time at which extravascular activity is maximal has been designated as the “equilibrium time.” When this point has been reached, the radioactivity remaining in the intravascular and extravascular spaces decreases slowly and exponentially in parallel fashion.
The administered radioactivity is eliminated almost entirely in the urine, only about 2 percent of the total dose ultimately appears in the feces.
The biologic half-life of iodinated I 125 albumin is dependent upon a number of factors, and published studies have varied considerably in their reporting of this figure. It has ranged, in the literature, from below 10 days to over 20 days. One important factor affecting the biologic half-life is the initial rate of excretion, and this depends in part on the quality of the iodinated I 125 albumin. With JEANATOPE, the biologic half-life in normal individuals has been reported to be approximately 14 days.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
In the use of any radioactive material, care should be taken to minimize radiation exposure to the patient and healthcare providers consistent with proper patient management.Radiopharmaceuticals should be used by or under control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or whether iodinated I 125 albumin affects fertility in males or females.Pregnancy
Iodine-125 crosses the placenta and can permanently impair fetal thyroid function. JEANATOPE should be administered to a pregnant woman only if clearly needed. Administration of an appropriate thyroid blocking agent is recommended before use of JEANATOPE in a pregnant woman to minimize the uptake of radioactive iodine for the woman and fetus (see DOSAGE AND ADMINISTRATION).There are no data on iodinated I 125 albumin use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted. All radiopharmaceuticals have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Nursing Mothers
Iodine-125 is present in human milk. There are no data on the effect of iodinated I 125 albumin on the breastfed infant or milk production. Because of the potential for serious adverse reactions in the breastfed infant, including transient hypothyroidism, advise that breastfeeding be discontinued and that breastfeeding not be initiated if a woman is exposed to JEANATOPE during pregnancy.Pediatric Use
Safety and effectiveness of JEANATOPE in pediatric patients have not been established.Geriatric Use
Clinical studies of JEANATOPE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. -
ADVERSE REACTIONS
The following adverse reactions have been identified with the use of iodinated I 125 albumin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity
-
DOSAGE AND ADMINISTRATION
Premedication
Administer 10 drops of Strong Iodine Solution, USP (e.g., Lugol’s Solution) three times daily, beginning at least 24 hours before administration of JEANATOPE and continue for 1 week or 2 weeks thereafter to minimize the uptake of iodine-125.Recommended Dosage
The recommended dose of JEANATOPE for total blood or plasma volume determination in adult patients is from 0.185 MBq to 1.85 MBq (5 microCi to 50 microCi) administered intravenously.When a procedure such as a blood volume determination is to be repeated, do not exceed 7.4 MBq (200 microCi) in any 1 week.
Administration Instructions
•Measure the patient dose using a suitable dose calibrator immediately prior to administration.
•Use a shielded syringe for withdrawing and injecting JEANATOPE.
•Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. JEANATOPE is colorless to very pale yellow solution. Do not use JEANATOPE if excessive coloration is present.
•The expiration date given on the JEANATOPE vial label pertains to the stability of JEANATOPE and not to the radioactivity level.Blood Volume Determination
Preparation of Reference Solution
1. Remove the amount from the vial to be used in the procedure identical in volume to the dose to be administered to the patient.
2. Prepare a reference solution using 0.9% Sodium Chloride Injection, USP as a diluent. The recommended dilution is 1:4,000 [Dilution Factor (DF) = 4,000].
3. Determine the radioactivity concentration (net cpm/mL) of the reference solution.
4. Assay the reference solution and the blood samples (Step 3 of Administration of Dose) using the same geometric configuration.Administration of Dose
1. Inject the dose into a large vein in patient’s arm. Measure the residual radioactivity in the syringe and needle.
2. Do not re-use the syringe. Dispose the syringe in accordance with the US Nuclear Regulatory Commission or Agreement State regulations pertaining to the disposal of radioactive waste.
3. At 5 minutes and 15 minutes after injecting the dose, withdraw blood samples from the patient’s other arm with a sterile heparinized syringe.Calculation of Blood Volume
1. Take a known aliquot from each blood sample and determine radioactivity concentration in net cpm/mL.
2. Plot the 5-minute and 15- minute sample counts (net cpm/mL) on semilog graph paper using the average count value of each sample and determine the radioactivity concentration at injection time (zero time) by drawing a straight line through the 15-minute and 5-minute points to zero time. The x ordinate of the graph is the sample withdrawal time and the logarithmic y ordinate is radioactivity concentration in net cpm/mL.
3. Calculate patient’s blood volume (in mL) using the following formula:
*DF: Dilution factor of reference solution
Sample Blood Volume Calculations
Volume of blood sample aliquot = 1 mL
Volume of reference solution aliquot = 1 mL
Net counts at zero time = 2,500
Net counts obtained from reference solution aliquot = 2,725
Dilution factor of reference solution = 4,000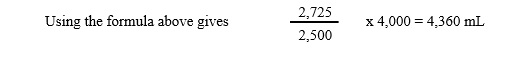
Serial Blood Voluma Determinations
•Use a low dose of JEANOTOPE to permit repetitions as often as required by clinical circumstances
•In each determination after the first one, correct the background radioactivity remaining in the blood from former determinations by subtracting the radioactivity concentration of a blood sample obtained before the injection of JEANATOPE (i.e., background blood sample) from the radioactivity concentration of a post-injection blood sample.Background Blood Sample
1. Prior to injecting JEANATOPE, withdraw background blood sample from large vein in patient’s arm with a sterile heparinized syringe.
2. Leaving needle in patient’s vein, detach syringe containing blood sample.
3. Attach syringe containing the dose of JEANATOPE to the indwelling needle and administer (see instructions under Blood Volume Determination, Administration of Dose).
4. Determine radioactivity concentration in net cpm/mL of aliquots taken from background and post-injection blood samples, and from the reference solution.Calculation of Blood Volume
Subtract the radioactivity concentration (net cpm/mL) per aliquot of the background blood sample from the radioactivity concentration per aliquot of the blood sample obtained after the injection of JEANATOPE. The formula for calculating each blood volume determination after the first one thus becomes:
*DF: Dilution factor of reference solution
Plasma Volume Determination
The procedure is essentially the same as that for blood volume determination, except that the blood sample drawn from the patient is centrifuged, the red blood cells are removed, and net cpm /mL of the plasma is determined. The formula for calculation of plasma volume, therefore is: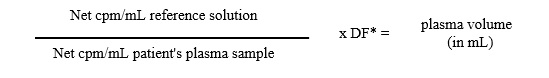
*DF: Dilution factor of reference solution
Radiation Dosimetry
The estimated absorbed radiation doses to an adult patient from an intravenous injection of 1.85 MBq (50 microCi) of JEANATOPE are shown in Table 4.
Method of Calculation: Hine GJ, Johnston RE: Absorbed Doses from Radionuclides, J. Nucl Med 11:468-469, 1970.
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
This radiopharmaceutical is licensed for distribution to facilities and persons licensed by the U.S. Nuclear Regulatory Commission or under an equivalent license issued by an Agreement State.
Iso-Tex Diagnostics, Inc. • U.S. License No. 2189
1511 Country Road 129 Alvin, TX 77511, U.S.A
(281) 482-1231 • FAX: (281) 482-1070Revised: 3/2023
Code 95-1732/Rev.002
- Packaging
-
INGREDIENTS AND APPEARANCE
JEANATOPE
iodinated i-125 albumin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50914-7733 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HUMAN SERUM ALBUMIN I-125 (UNII: 68WQQ3N9TI) (HUMAN SERUM ALBUMIN I-125 - UNII:68WQQ3N9TI) HUMAN SERUM ALBUMIN I-125 1000 uCi in 1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50914-7733-5 1 in 1 CONTAINER 03/09/2022 1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA017836 03/09/2022 Labeler - Iso-Tex Diagnostics, Inc. (181202995) Establishment Name Address ID/FEI Business Operations Iso-Tex Diagnostics, Inc. 181202995 manufacture(50914-7733)



