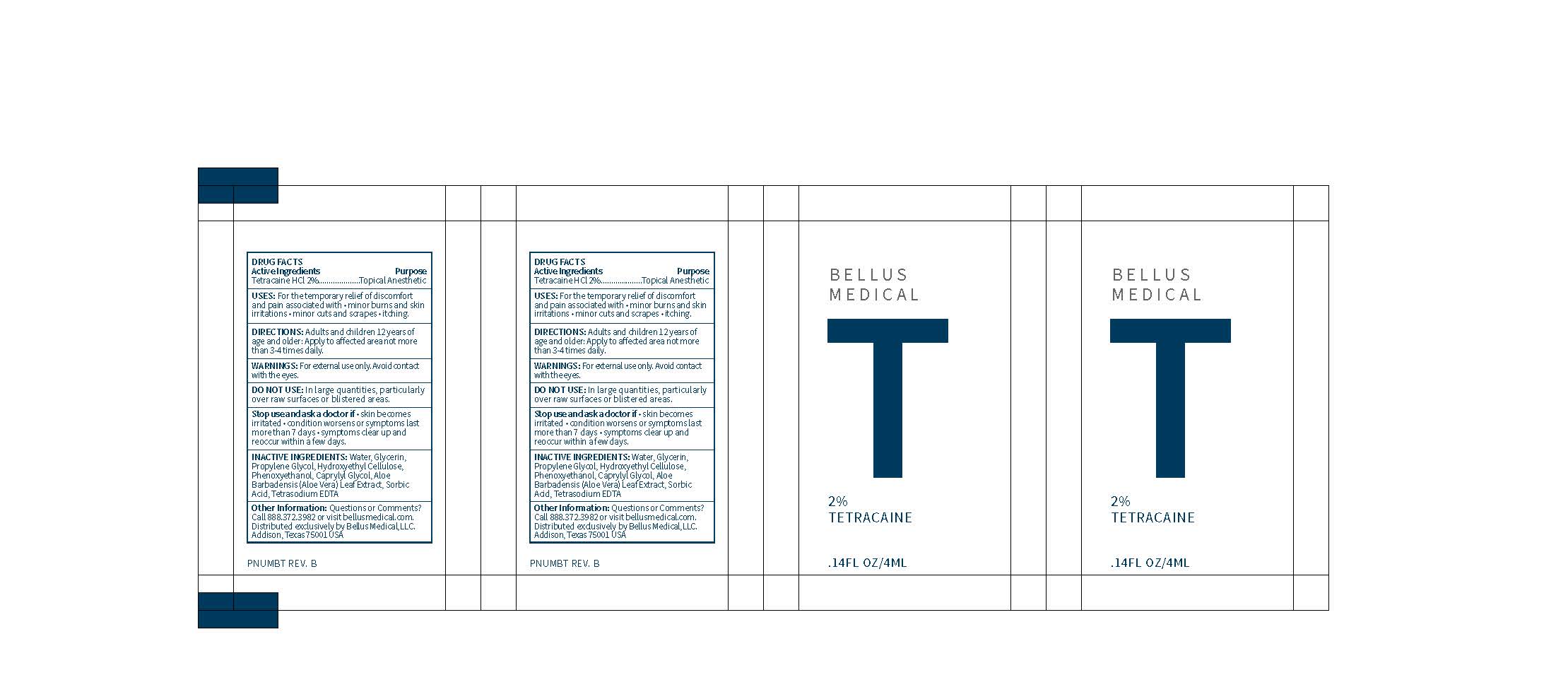
Label: TETRACAINE gel
- NDC Code(s): 71888-103-01, 71888-103-02
- Packager: Bellus Medical, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
WARNINGS
Warnings : For external use only. Avoid contact with the eyes.
DO NOT USE : In large quantities, particularly over raw surfaces and blistered areas.
Stop use and ask a doctor if:
- Skin becomes irritated
- Condition worsens or symptoms last more than 7 days
- Symptoms clear up and reoccur within a few days.
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TETRACAINE
tetracaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71888-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACAINE HYDROCHLORIDE (UNII: 5NF5D4OPCI) (TETRACAINE - UNII:0619F35CGV) TETRACAINE HYDROCHLORIDE 20 mg in 1000 mL Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) CAPRYLYL GLYCOL (UNII: 00YIU5438U) EDETATE SODIUM (UNII: MP1J8420LU) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) SORBIC ACID (UNII: X045WJ989B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71888-103-02 12 in 1 BOX 06/01/2017 1 NDC:71888-103-01 4 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2017 Labeler - Bellus Medical, LLC (005677967) Registrant - Bellus Medical, LLC (005677967)