Label: FORMULA 3- tolnaftate solution
- NDC Code(s): 54291-036-01
- Packager: Canadian Custom Packaging Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- FORMULA 3
- Active ingredient:
- Purpose
- Uses:
-
Warnings
For external use only.
Do not use on children under 2 years of age unless directed by a doctor.
When using this product avoid contact with eyes.
Stop use and ask a doctor if
- When using for athlete's foot or ringworm: If irritation occurs or if there is no improvement within 4 weeks, discontinue use and consult a doctor.
- When using for the prevention of athlete's foot: If irritation occurs,discontinue use and consult a doctor.
If pregnant or breastfeeding ask a health professional before use.
- Keep out of reach of children.
-
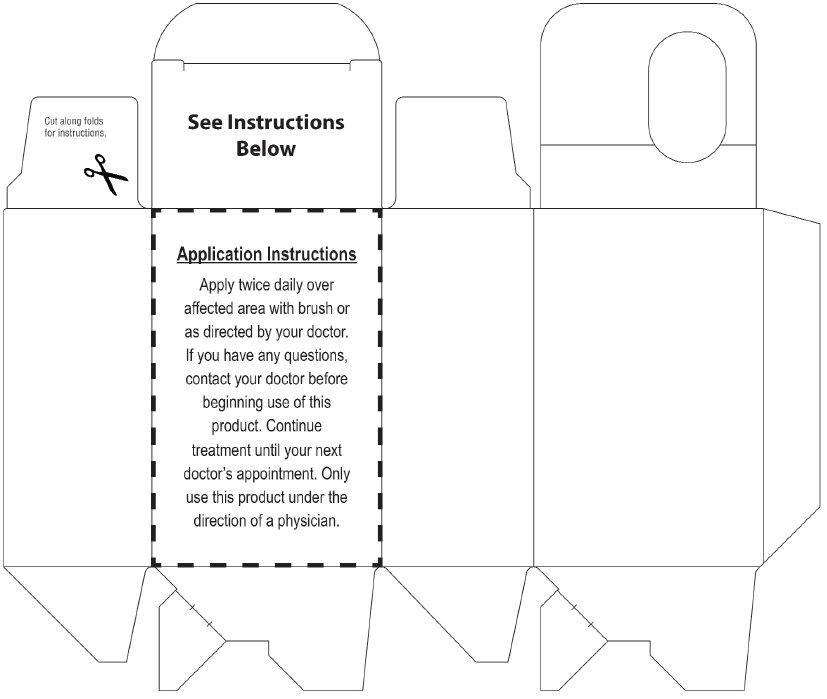
Directions
For treatment of athlete's foot and ringworm
- Clean the affected area and dry thoroughly. Apply a thin layer of the product over affected area twice daily (morning and night) or as directed by a doctor.
- Supervise children in the use of this product.
- For athlete's foot: Pay special attention to spaces between the toes; wear well fitting, ventilated shoes, and change shoes and socks at least once daily.
- For athlete's foot and ringworm, use daily for 4 weeks.
- If condition persists longer, consult a doctor. This product is not effective on scalp or nail.
To prevent athlete's foot:
- Clean the feet and dry thoroughly.
- Apply a thin layer of the product to the feet once or twice daily (morning and/or night)
- Supervise children in the use of this product.
- Pay special attention to spaces between the toes; wear well fitting, ventilated shoes, and change shoes and socks at least once daily.
- Inactive ingredients
- FORMULA 3 BRAND Antifungal Tolnaftate 1% Antifungal Solution 15ml (49406-003-15)
-
INGREDIENTS AND APPEARANCE
FORMULA 3
tolnaftate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54291-036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength APRICOT KERNEL OIL (UNII: 54JB35T06A) RICE BRAN OIL (UNII: LZO6K1506A) SESAME OIL (UNII: QX10HYY4QV) SAFFLOWER OIL (UNII: 65UEH262IS) JOJOBA OIL (UNII: 724GKU717M) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLPARABEN (UNII: 3QPI1U3FV8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54291-036-01 1 in 1 BOX 10/30/2012 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 10/30/2012 Labeler - Canadian Custom Packaging Company (207062514) Registrant - Canadian Custom Packaging Company (207062514) Establishment Name Address ID/FEI Business Operations Canadian Custom Packaging Company 207062514 manufacture(54291-036)