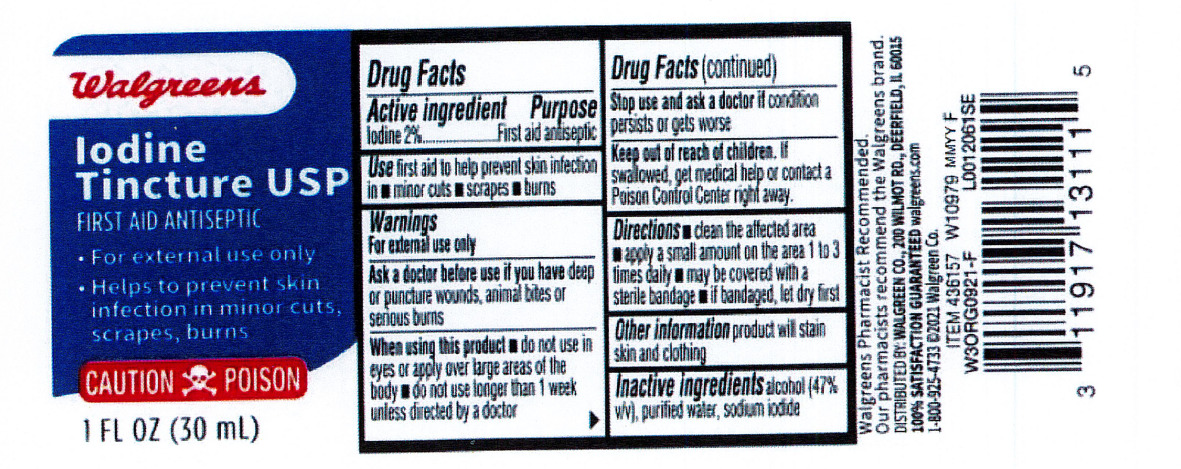
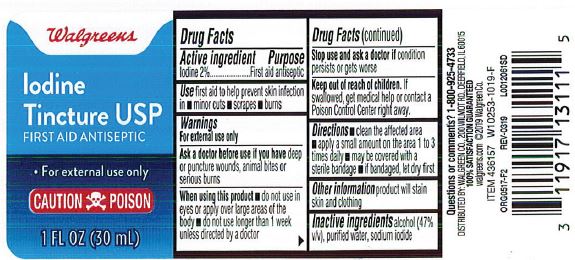
Label: IODINE TINCTURE MILD- iodine and sodium iodide and alcohol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 0395-9118-91 - Packager: Humco Holding Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
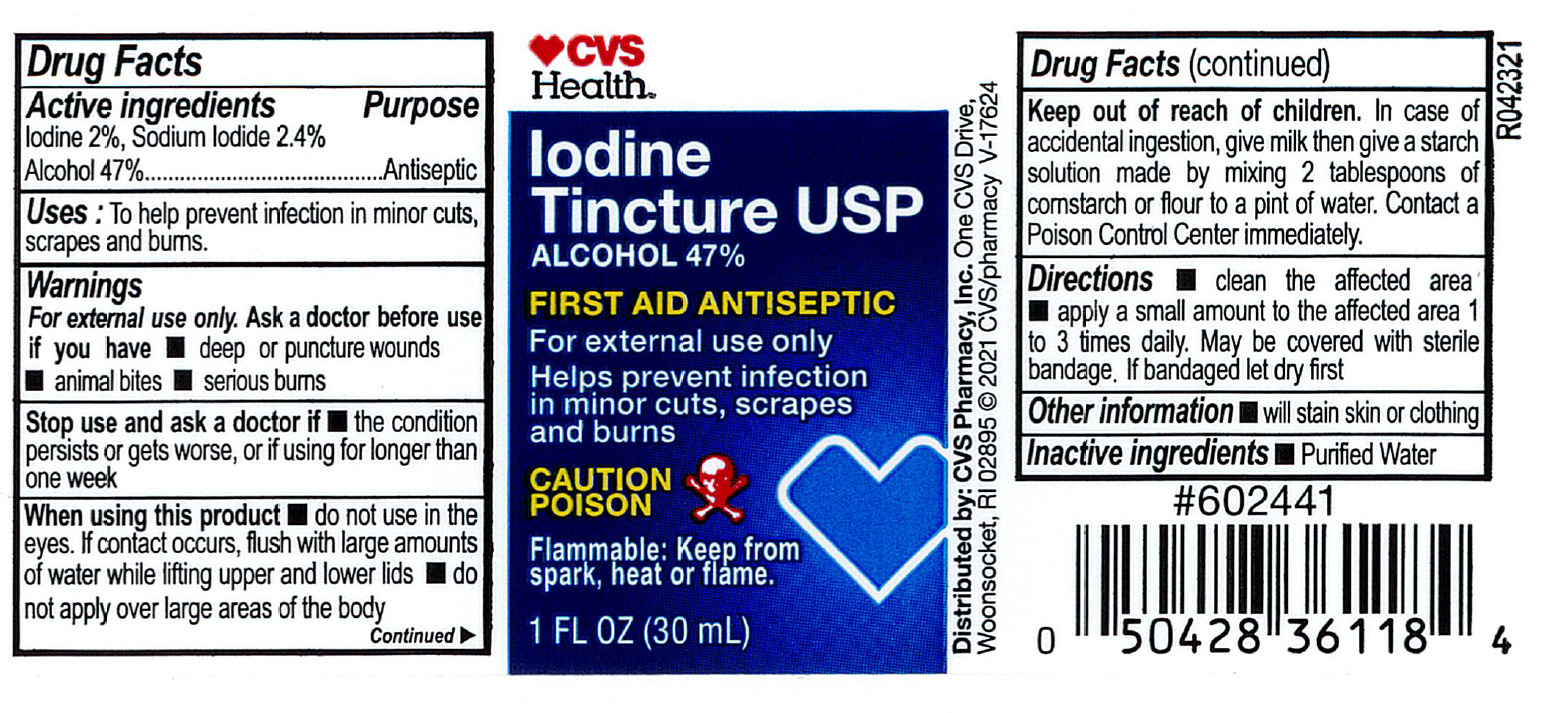
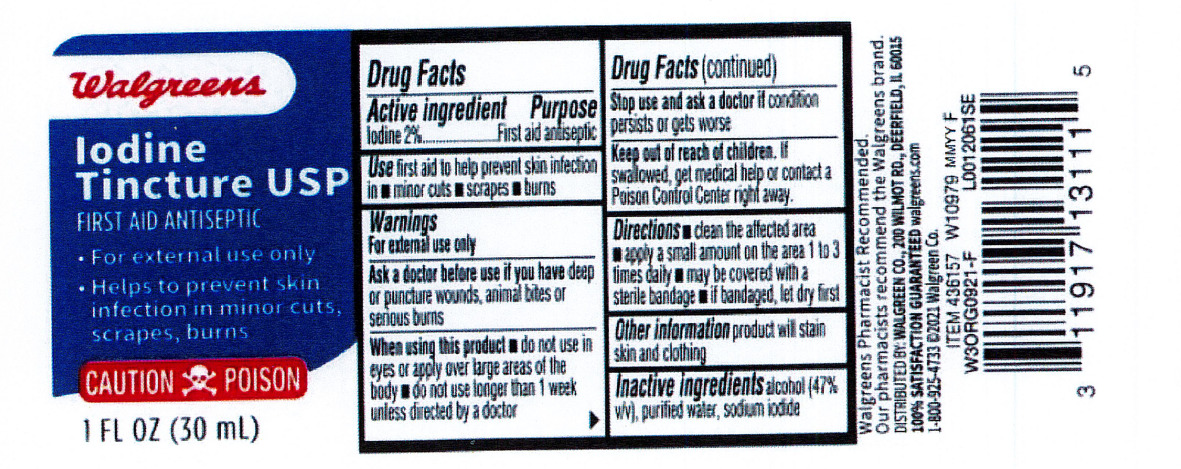
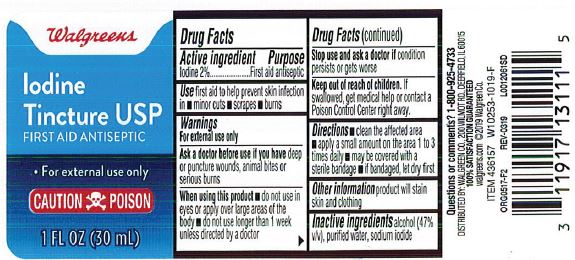
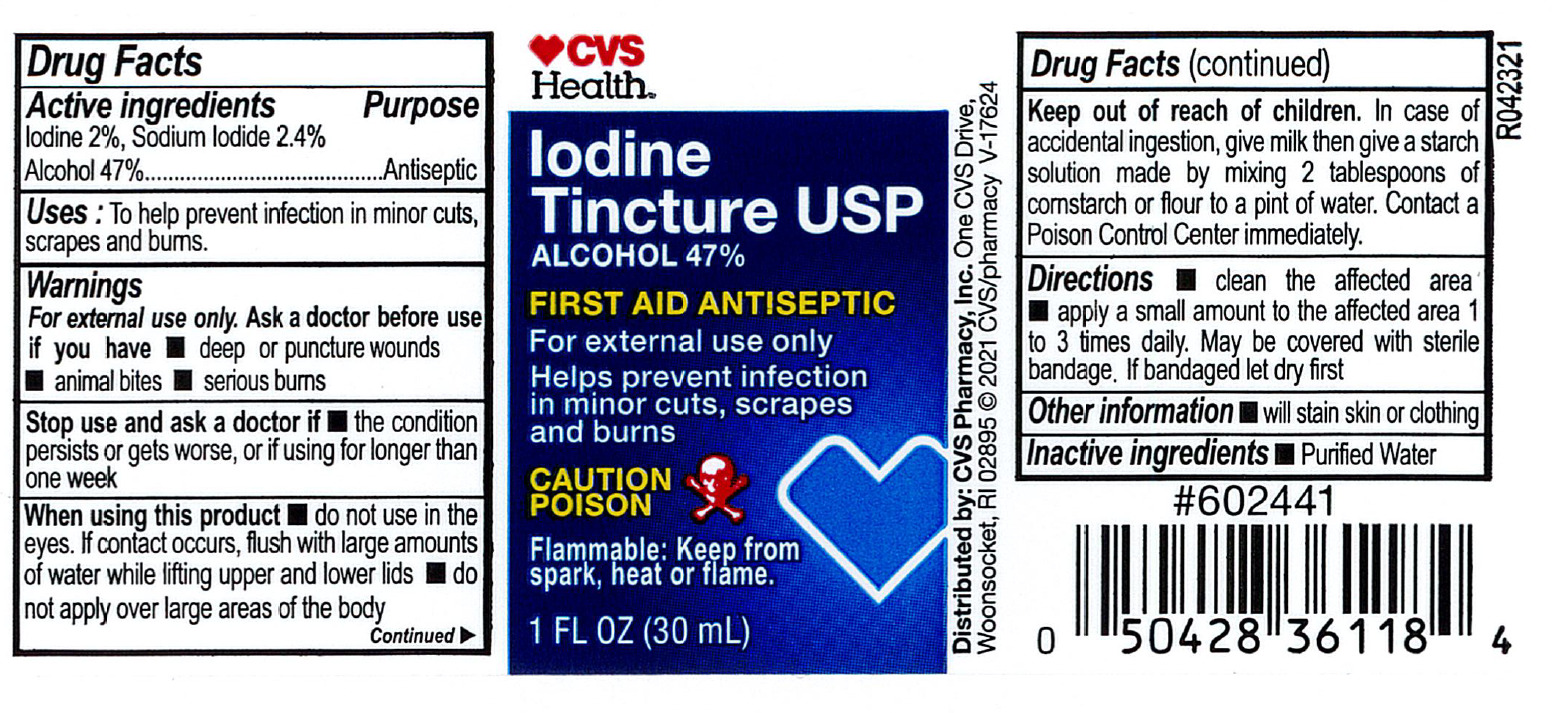
- Active ingredient
- Purpose
- Active ingredient
- Purpose
- Active ingredient
- Purpose
- Indications
-
Warnings
For external use only.
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
- Flammable: Keep away from sparks heat and flame
Stop use and consult doctor if
- the condition persists or gets worse, or if using for longer than one week
- deep or puncture wounds
- Directions
- Other information
- Inactive ingredient
- Good Neighbor Label
- Leader Label
- Quality Choice Label
- Sunmark Label
- Premier Value Label
- DDM Label
- De La Cruz Label
- CVS Label
- Harris Teeter Label
- Kroger Label
- Safeway Label
- Vida Mia Label
- Swan Label
- Good Sense Label
- Walgreen Label
- Health Mart Label
- Equaline Label
-
INGREDIENTS AND APPEARANCE
IODINE TINCTURE MILD
iodine and sodium iodide and alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0395-9118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 20 mg in 1 mL SODIUM IODIDE (UNII: F5WR8N145C) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 20.4 mg in 1 mL ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 470 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0395-9118-91 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/14/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/01/1979 Labeler - Humco Holding Group, Inc. (825672884) Registrant - Humco Holding Group, Inc. (825672884) Establishment Name Address ID/FEI Business Operations Humco Holding Group, Inc. 825672884 manufacture(0395-9118) , analysis(0395-9118) , pack(0395-9118) , label(0395-9118)