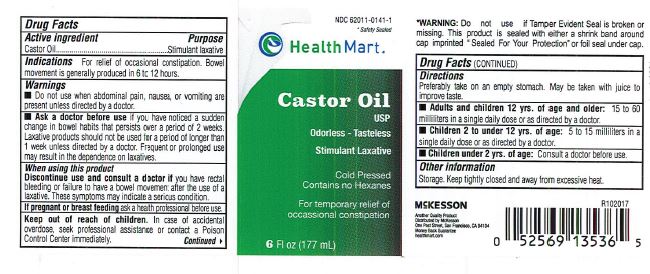
Label: HUMCO CASTOR OIL- castor oil liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 0395-9113-16, 0395-9113-92, 0395-9113-96 - Packager: Humco Holding Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
- Warings
- Ask a doctor before use
- When using this product
- If pregnant or breast feeding
- Keep out of reach of children.
-
Directions
Preferably take on an empty stomach. May be taken with juice to improve taste.
Adults and children 12 yrs. of age and older: 15 to 60 milliliters in a single daily dose or as directed by a doctor.
Children 2 to under 12 yrs. of age: 5 to 15 milliliters in a single dose or as directed by a doctor.
Children under 2 yrs. of age: Consult a doctor before use.
- WARNINGS:
- Other Information
- Inactive Ingredient
- GNP Label
- Leader Label
- Quality Choice Label
- Sunmark Label
- Premier Value Label
- DDM Label
- Rite Aid Label
- Harris Teeter Label
- Vida Mia Label
- Heamth Mart Label
- Top Care Label
-
INGREDIENTS AND APPEARANCE
HUMCO CASTOR OIL
castor oil liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0395-9113 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CASTOR OIL (UNII: D5340Y2I9G) (CASTOR OIL - UNII:D5340Y2I9G) CASTOR OIL 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0395-9113-16 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/13/2017 2 NDC:0395-9113-96 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/13/2017 3 NDC:0395-9113-92 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/13/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 01/01/2008 Labeler - Humco Holding Group, Inc. (825672884) Registrant - Humco Holding Group, Inc. (825672884) Establishment Name Address ID/FEI Business Operations Humco Holding Group, Inc. 825672884 manufacture(0395-9113) , analysis(0395-9113) , pack(0395-9113) , label(0395-9113)