Label: QUFLORA PEDIATRIC DROPS- vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolic acid, cyanocobalamin, magnesium oxide, cupric sulfate, and sodium fluoride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 15370-100-50 - Packager: CarWin Pharmaceutical Associates, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 3, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
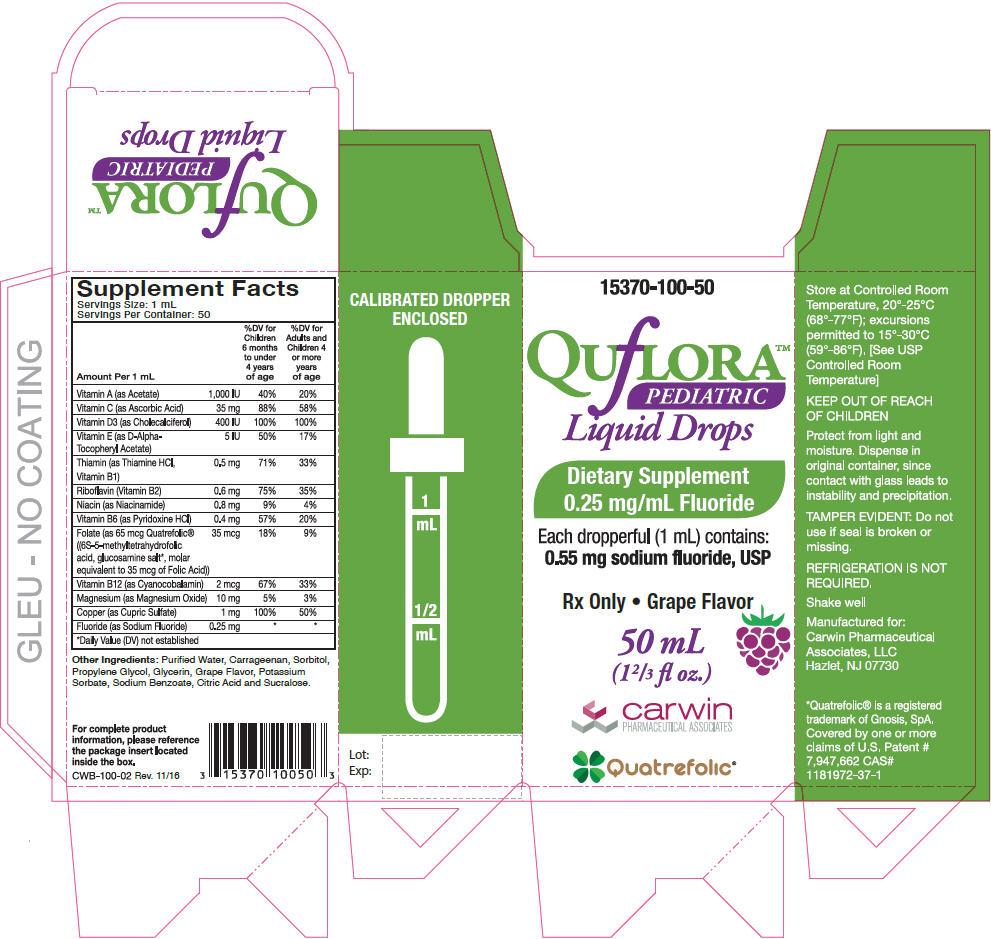
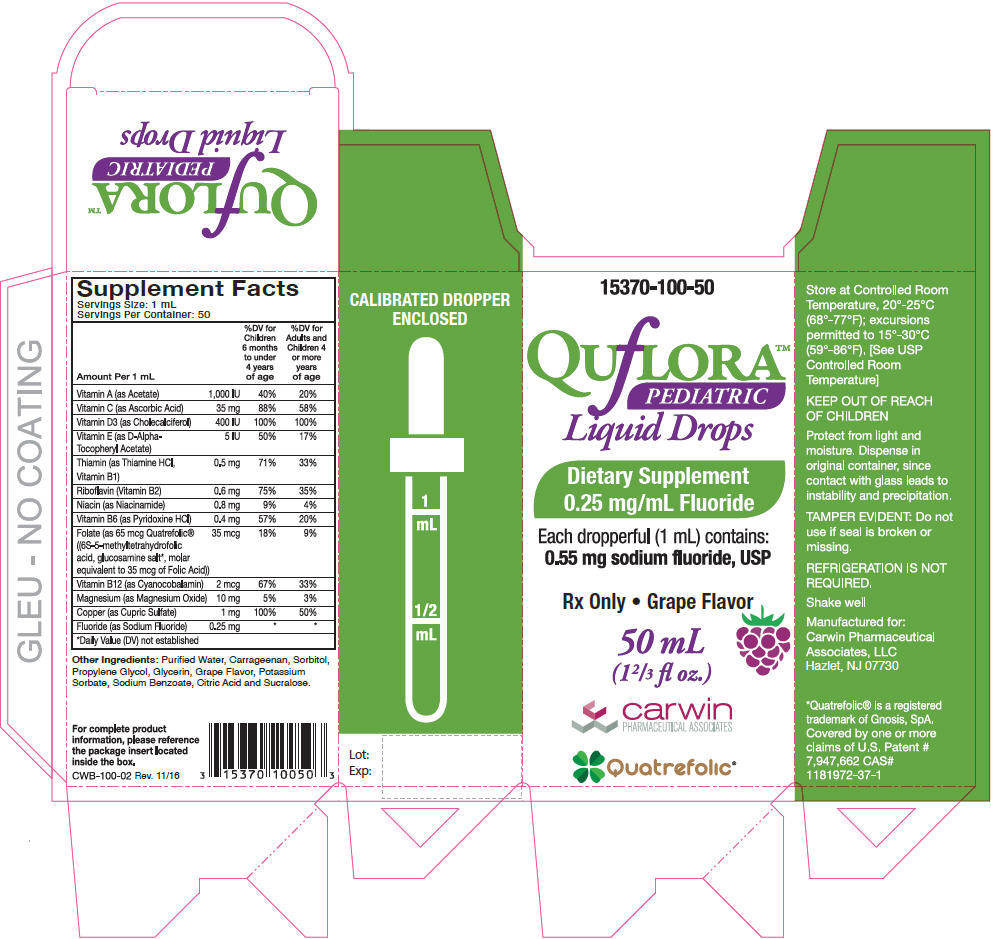
Supplement Facts
Serving Size: 1 mL
Servings Per Container: 50Amount Per 1 mL % Daily Value Children 6 months to under 4 years of age % Daily Value for Adults and Children 4 or more years of age - *
- Daily Value (DV) not established
Vitamin A (as Acetate) 1,000 IU 40% 20% Vitamin C (as Ascorbic Acid) 35 mg 88% 58% Vitamin D3 (as Cholecalciferol) 400 IU 100% 100% Vitamin E (as D-Alpha-Tocopheryl Acetate) 5 IU 50% 17% Thiamin (as Thiamine HCl, Vitamin B1) 0.5 mg 71% 33% Riboflavin (Vitamin B2) 0.6 mg 75% 35% Niacin (as Niacinamide) 0.8 mg 9% 4% Vitamin B6 (as Pyridoxine HCl) 0.4 mg 57% 20% Folate (as 65 mcg Quatrefolic® ((6S-5-methyltetrahydrofolic acid, glucosamine salt*, molar equivalent to 35 mcg of Folic Acid)) 35 mcg 18% 9% Vitamin B12 (as Cyanocobalamin) 2 mcg 67% 33% Magnesium (as Magnesium Oxide) 10 mg 5% 3% Copper (as Cupric Sulfate) 1 mg 100% 50% Fluoride (as Sodium Fluoride) 0.25 mg * * Other Ingredients: Purified Water, Carrageenan, Sorbitol, Propylene Glycol, Glycerin, Grape Flavor, Potassium Sorbate, Sodium Benzoate, Citric Acid and Sucralose.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Do not use this product if you are allergic to any of the ingredients. The suggested dose should not be exceeded, since dental fluorosis may result from continued ingestion of large amounts of fluoride. Do not eat or drink dairy products within one hour of fluoride administration since these may decrease effectiveness.
Folic Acid
Folic Acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
- WARNINGS
-
DOSAGE AND ADMINISTRATION
See schedule below to determine dosage. Administer orally as prescribed by a healthcare practitioner. Use with calibrated dropper for measuring doses.
Fluoride Ion Level In Drinking Water (ppm)* Age Less than 0.3ppm 0.3-0.6 ppm Greater than 0.6 ppm Birth-6 months None None None 6 months-3 years 0.25 mg/day† None None 3-6 years 0.50 mg/day† 0.25 mg/day None 6-16 years 1.0 mg/day 0.50 mg/day None Dietary Fluoride Supplement dosing schedule approved by the American Dental Association (ADA), American Academy of Pediatrics (AAP) & American Academy of Pediatric Dentistry (AAPD).
- DESCRIPTION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
QUFLORA PEDIATRIC DROPS
vitamin a acetate, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolic acid, cyanocobalamin, magnesium oxide, cupric sulfate, and sodium fluoride liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:15370-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1000 [iU] in 1 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 35 mg in 1 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] in 1 mL .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 5 [iU] in 1 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 0.5 mg in 1 mL RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 0.6 mg in 1 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 0.8 mg in 1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 0.4 mg in 1 mL LEVOMEFOLIC ACID (UNII: 8S95DH25XC) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 35 ug in 1 mL CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 2 ug in 1 mL MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 10 mg in 1 mL CUPRIC SULFATE (UNII: LRX7AJ16DT) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 1 mg in 1 mL SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARRAGEENAN (UNII: 5C69YCD2YJ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GRAPE (UNII: 6X543N684K) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Product Characteristics Color BROWN Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15370-100-50 1 in 1 CARTON 07/15/2014 1 50 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 07/15/2014 Labeler - CarWin Pharmaceutical Associates, LLC (079217215)