Label: SWEET CHEEKS- zinc oxide ointment
- NDC Code(s): 70983-021-01, 70983-021-02
- Packager: Saje Natural Business Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
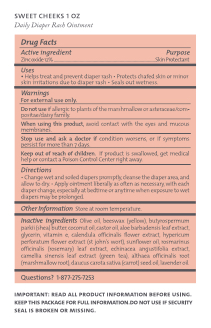
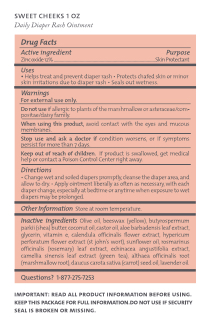
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Olive oil, beeswax (yellow), butyrospermum parkii (shea) butter, coconut oil, castor oil, aloe barbadensis leaf extract, glycerin, vitamin e, calendula officinalis flower extract, hypericum perforatum flower extract (st john's wort), sunflower oil, rosmarinus officinalis (rosemary) leaf extract, echinacea angustifolia extract, camellia sinensis leaf extract (green tea), althaea officinalis root (marshmallow root), daucus carota sativa (carrot) seed oil, lavender oil
- Questions?
- Principal display panel information
- Sweet cheeks daily diaper rash ointment-Outer label
-
INGREDIENTS AND APPEARANCE
SWEET CHEEKS
zinc oxide ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70983-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 9 g in 75 g Inactive Ingredients Ingredient Name Strength SHEA BUTTER (UNII: K49155WL9Y) COCONUT OIL (UNII: Q9L0O73W7L) YELLOW WAX (UNII: 2ZA36H0S2V) CASTOR OIL (UNII: D5340Y2I9G) SUNFLOWER OIL (UNII: 3W1JG795YI) TOCOPHEROL (UNII: R0ZB2556P8) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) LAVENDER OIL (UNII: ZBP1YXW0H8) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) OLIVE OIL (UNII: 6UYK2W1W1E) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) ROSEMARY (UNII: IJ67X351P9) ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) CARROT SEED OIL (UNII: 595AO13F11) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70983-021-01 1 in 1 BOX 02/15/2018 1 75 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:70983-021-02 30 g in 1 JAR; Type 0: Not a Combination Product 02/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/15/2018 Labeler - Saje Natural Business Inc. (080465432)