Label: ocupress- carteolol hydrochloride solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 58768-001-01, 58768-001-02, 58768-001-04 - Packager: Novartis Ophthalmics
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 19, 2006
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Ocupress® (carteolol hydrochloride ophthalmic solution), 1%, is a nonselective beta-adrenoceptor blocking agent for ophthalmic use.
The chemical name for carteolol hydrochloride is (±)–5–[3–[(1,1–dimethylethyl) amino]–2 hydroxypropoxy]–3, 4–dihydro–2(1H)–quinolinone monohydrochloride. The structural formula is as follows:
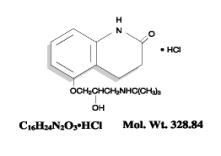
Each mL contains 10 mg carteolol HCl and the inactive ingredients – Benzalkonium chloride 0.05 mg (0.005%) as a preservative; sodium chloride; sodium phosphate, dibasic; sodium phosphate, monobasic; and water for injection, USP. The product has a pH of 6.2 to 7.2
-
CLINICAL PHARMACOLOGY
Carteolol HCl is a nonselective beta-adrenergic blocking agent with associated intrinsic sympathomimetic activity and without significant membrane-stabilizing activity.
Ocupress (carteolol HCl) reduces normal and elevated intraocular pressure (IOP) whether or not accompanied by glaucoma. The exact mechanism of the ocular hypotensive effect of beta-blockers has not been definitely demonstrated.
In general, beta-adrenergic blockers reduce cardiac output in patients in good and poor cardiovascular health. In patients with severe impairment of myocardial function, beta-blockers may inhibit the sympathetic stimulation necessary to maintain adequate cardiac function. Beta-adrenergic blockers may also increase airway resistance in the bronchi and bronchioles due to unopposed parasympathetic activity.
Given topically twice daily in controlled domestic clinical trials ranging from 1.5 to 3 months, Ocupress produced a median percent reduction of IOP 22% to 25%. No significant effects were noted on corneal sensitivity, tear secretion, or pupil size.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Ocupress Ophthalmic Solution is contraindicated in those individuals with bronchial asthma or with a history of bronchial asthma, or severe chronic obstructive pulmonary disease (see WARNINGS); sinus bradycardia; second- and third-degree atrioventricular block; overt cardiac failure (see WARNINGS); cardiogenic shock; or hypersensitivity to any component of this product.
-
WARNINGS
Ocupress Ophthalmic Solution has not been detected in plasma following ocular instillation. However, as with other topically applied ophthalmic preparations, Ocupress may be absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely death in association with cardiac failure, have been reported with topical application of beta-adrenergic blocking agents (see CONTRAINDICATIONS).
Cardiac Failure
Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more severe failure.
In Patients Without a History of Cardiac Failure
Continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of cardiac failure, Ocupress should be discontinued.
Non-allergic Bronchospasm
In patients with non-allergic bronchospasm or with a history of non-allergic bronchospasm (e.g., chronic bronchitis, emphysema), Ocupress should be administered with caution since it may block bronchodilation produced by endogenous and exogenous catecholamine stimulation of beta2 receptors.
Major Surgery
The necessity or desirability of withdrawal of beta-adrenergic blocking agents prior to major surgery is controversial. Beta-adrenergic receptor blockade impairs the ability of the heart to respond to beta-adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta-adrenergic receptor blocking agents have been subject to protracted severe hypotension during anesthesia. For these reasons, in patients undergoing elective surgery, gradual withdrawal of beta-adrenergic receptor blocking agents may be appropriate.
If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of such agonists as isoproterenol, dopamine, dobutamine or levarterenol (see OVERDOSAGE).
Diabetes Mellitus
Beta-adrenergic blocking agents should be administered with caution in patients subject to spontaneous hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents. Beta-adrenergic receptor blocking agents may mask the signs and symptoms of acute hypoglycemia.
-
PRECAUTIONS
General
Ocupress Ophthalmic Solution should be used with caution in patients with known hypersensitivity to other beta-adrenoceptor blocking agents.
Use with caution in patients with known diminished pulmonary function.
In patients with angle-closure glaucoma, the immediate objective of treatment is to reopen the angle. This requires constricting the pupil with a miotic. Ocupress has little or no effect on the pupil. When Ocupress is used to reduce elevated intraocular pressure in angle-closure glaucoma, it should be used with a miotic and not alone.
Information to the Patient
For topical use only. To prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use. Protect from light.
Risk from Anaphylactic Reaction
While taking beta-blockers, patients with a history of atopy or a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated accidental, diagnostic, or therapeutic challenge with such allergens. Such patients may be unresponsive to the usual doses of epinephrine used to treat anaphylactic reactions.
Muscle Weakness
Beta-adrenergic blockade has been reported to potentiate muscle weakness consistent with certain myasthenic symptoms (e.g., diplopia, ptosis and generalized weakness).
Drug Interactions
Ocupress should be used with caution in patients who are receiving a beta-adrenergic blocking agent orally, because of the potential for additive effects on systemic beta-blockade.
Close observation of the patient is recommended when a beta-blocker is administered to patients receiving catecholamine-depleting drugs such as reserpine, because of possible additive effects and the production of hypotension and/or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carteolol hydrochloride did not produce carcinogenic effects at doses up to 40 mg/kg/day in two-year oral rat and mouse studies. Tests of mutagenicity, including the Ames Test, recombinant (rec)-assay, in vivo cytogenetics and dominant lethal assay demonstrated no evidence for mutagenic potential. Fertility of male and female rats and male and female mice was unaffected by administration of carteolol hydrochloride dosages up to 150 mg/kg/day.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Carteolol hydrochloride increased resorptions and decreased fetal weights in rabbits and rats at maternally toxic doses approximately 1052 and 5264 times the maximum recommended human oral dose (10 mg/70 kg/day), respectively. A dose-related increase in wavy ribs was noted in the developing rat fetus when pregnant females received daily doses of approximately 212 times the maximum recommended human oral dose. No such effects were noted in pregnant mice subjected to up to 1052 times the maximum recommended human oral dose. There are no adequate and well-controlled studies in pregnant women. Ocupress (carteolol hydrochloride) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
ADVERSE REACTIONS
The following adverse reactions have been reported in clinical trials with Ocupress Ophthalmic Solution:
Ocular
Transient eye irritation, burning, tearing, conjunctival hyperemia and edema occurred in about 1 of 4 patients. Ocular symptoms including blurred and cloudy vision, photophobia, decreased night vision, and ptosis and ocular signs including blepharoconjunctivitis, abnormal corneal staining, and corneal sensitivity occurred occasionally.
Systemic
As is characteristic of nonselective adrenergic blocking agents, Ocupress may cause bradycardia and decreased blood pressure (see WARNINGS). The following systemic events have occasionally been reported with the use of Ocupress: cardiac arrhythmia, heart palpitation, dyspnea, asthenia, headache, dizziness, insomnia, sinusitis, and taste perversion.
The following additional adverse reactions have been reported with ophthalmic use of beta1 and beta2 (nonselective) adrenergic receptor blocking agents:
Body As a Whole: Headache
Cardiovascular: Arrhythmia, syncope, heart block, cerebral vascular accident, cerebral ischemia, congestive heart failure, palpitation (see WARNINGS).
Digestive: Nausea
Psychiatric: Depression
Skin: Hypersensitivity, including localized and generalized rash
Respiratory: Bronchospasm (predominantly in patients with pre-existing bronchospastic disease), respiratory failure (see WARNINGS)
Endocrine: Masked symptoms of hypoglycemia in insulin-dependent diabetics (see WARNINGS)
Special Senses: Signs and symptoms of keratitis, blepharoptosis, visual disturbances including refractive changes (due to withdrawal of miotic therapy in some cases), diplopia, ptosis.
Other reactions associated with the oral use of nonselective adrenergic receptor blocking agents should be considered potential effects with ophthalmic use of these agents.
-
OVERDOSAGE
No specific information on emergency treatment of overdosage in humans is available. Should accidental ocular overdosage occur, flush eye(s) with water or normal saline. The most common effects expected with overdosage of a beta-adrenergic blocking agent are bradycardia, bronchospasm, congestive heart failure and hypotension.
In case of ingestion, treatment with Ocupress should be discontinued and gastric lavage considered. The patient should be closely observed and vital signs carefully monitored. The prolonged effects of carteolol must be considered when determining the duration of corrective therapy. On the basis of the pharmacologic profile, the following additional measures should be considered as appropriate:
Symptomatic Sinus Bradycardia or Heart Block: Administer atropine. If there is no response to vagal blockade, administer isoproterenol cautiously.
Bronchospasm: Administer a beta2-stimulating agent such as isoproterenol and/or a theophylline derivative.
Congestive Heart Failure: Administer diuretics and digitalis glycosides as necessary.
Hypotension: Administer vasopressors such as intravenous dopamine, epinephrine or norepinephrine bitartrate.
-
DOSAGE AND ADMINISTRATION
The usual dose is one drop of Ocupress Ophthalmic Solution, 1%, in the affected eye(s) twice a day.
If the patient’s IOP is not at a satisfactory level on this regimen, concomitant therapy with pilocarpine and other miotics, and/or epinephrine or dipivefrin, and/or systemically administered carbonic anhydrase inhibitors, such as acetazolamide, can be instituted.
-
HOW SUPPLIED
Ocupress Ophthalmic Solution, 1%, is supplied as a sterile ophthalmic solution in plastic dispenser bottles of 5 mL (NDC 58768-001-01), 10 mL (NDC 58768-001-02) and 15 mL (NDC 58768-001-04).
Store at 15° to 25°C (59° to 77°F) (room temperature) and protect from light.
Rx Only
Licensed under U.S. Patent Nos. 3910924 and 4309432.
Made in Canada by:
CIBA Vision Sterile Mfg.,
Mississauga, Ontario L5N 2X5
For: Novartis Ophthalmics, Duluth, Georgia 30097
I6138-B
-
INGREDIENTS AND APPEARANCE
OCUPRESS
carteolol hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58768-001 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength carteolol hydrochloride (UNII: 4797W6I0T4) (carteolol - UNII:8NF31401XG) 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium chloride () 0.05 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) sodium phosphate, dibasic (UNII: GR686LBA74) sodium phosphate, monobasic (UNII: 3980JIH2SW) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58768-001-01 5 mL in 1 BOTTLE, DISPENSING 2 NDC:58768-001-02 10 mL in 1 BOTTLE, DISPENSING 3 NDC:58768-001-04 15 mL in 1 BOTTLE, DISPENSING Labeler - Novartis Ophthalmics
