Label: TRIPLE WORMER- pyrantel pamoate and praziquantel tablet, chewable
- NDC Code(s): 30798-185-61, 30798-185-71
- Packager: Durvet, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 20, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Package contents:
-
Drug Facts
Active Ingredients
(in each chewable):pyrantel pamoate (30 mg)
and praziquantel (30 mg)
Purpose: De-wormer for Small Dogs and Puppies Only (6.0 to 25 pounds).
Uses: For the treatment and control of:
• Roundworms (Toxocara canis, Toxascaris leonina)
• Hookworms (Ancylostoma caninum, Ancylostoma braziliense, and Uncinaria stenocephala)
• Tapeworms (Dipylidium caninum, Taenia pisiformis)
-
Human Warning:
Keep this and all medication out of the reach of children.
To obtain product information, including a Safety Data Sheet (SDS), call 1-800-821-5570.When Using This Product:
• Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.• Do not de-worm a dog or puppy that is sick. Consult a veterinarian for diagnosis of the illness.
• Triple Wormer
Flavored Chewables are safe for use in puppies 12 weeks or older and adult dogs.Safety in breeding dogs and pregnant bitches has not been tested.
- You May Notice:
-
Directions:
Each flavored chewable contains 30 mg of pyrantel pamoate and 30 mg of praziquantel. The dose for each drug is 2.27 mg per pound of body weight (5 mg/kg). Please refer to the following dosing table for help finding the right dose for your dog.
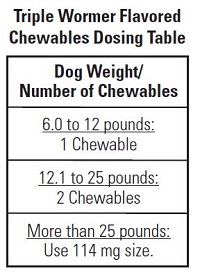
• You should weigh your dog to make sure you are giving the right dose.
• Triple Wormer Flavored Chewables are palatable if offered by hand. If your dog does not voluntarily eat the chewable, you can hide the chewable in a small amount of food or place it in the back of the dog's mouth for forced swallowing.
• Make sure that the dog eats the complete dose.
• Watch your dog for a few minutes after dosing to make sure the chewable is not rejected. -
Other Information:Recommended De-Worming Schedule:
Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism. De-worming schedules may vary depending on the climate where you live and the activity of your dog.
Re-treatment: Re-treatment of your dog may be necessary as determined
by laboratory fecal examination and/or if your dog is living where re-infections are likely to occur. Consult your veterinarian for assistance in the diagnosis and prevention of re-infection. In case of re-infection with tapeworms (Dipylidium caninum), consult your veterinarian for advice on how to remove fleas from thedog and the environment. - SPL UNCLASSIFIED SECTION
- STORAGE AND HANDLING
- Questions? Comments?
- Principle Display Panel- 2 Tablet Package
-
INGREDIENTS AND APPEARANCE
TRIPLE WORMER
pyrantel pamoate and praziquantel tablet, chewableProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:30798-185 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pyrantel pamoate (UNII: 81BK194Z5M) (pyrantel - UNII:4QIH0N49E7) pyrantel 30 mg praziquantel (UNII: 6490C9U457) (praziquantel - UNII:6490C9U457) praziquantel 30 mg Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30798-185-61 2 in 1 BOX, UNIT-DOSE 2 NDC:30798-185-71 12 in 1 BOX, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141261 03/06/2010 Labeler - Durvet, Inc. (056387798)


