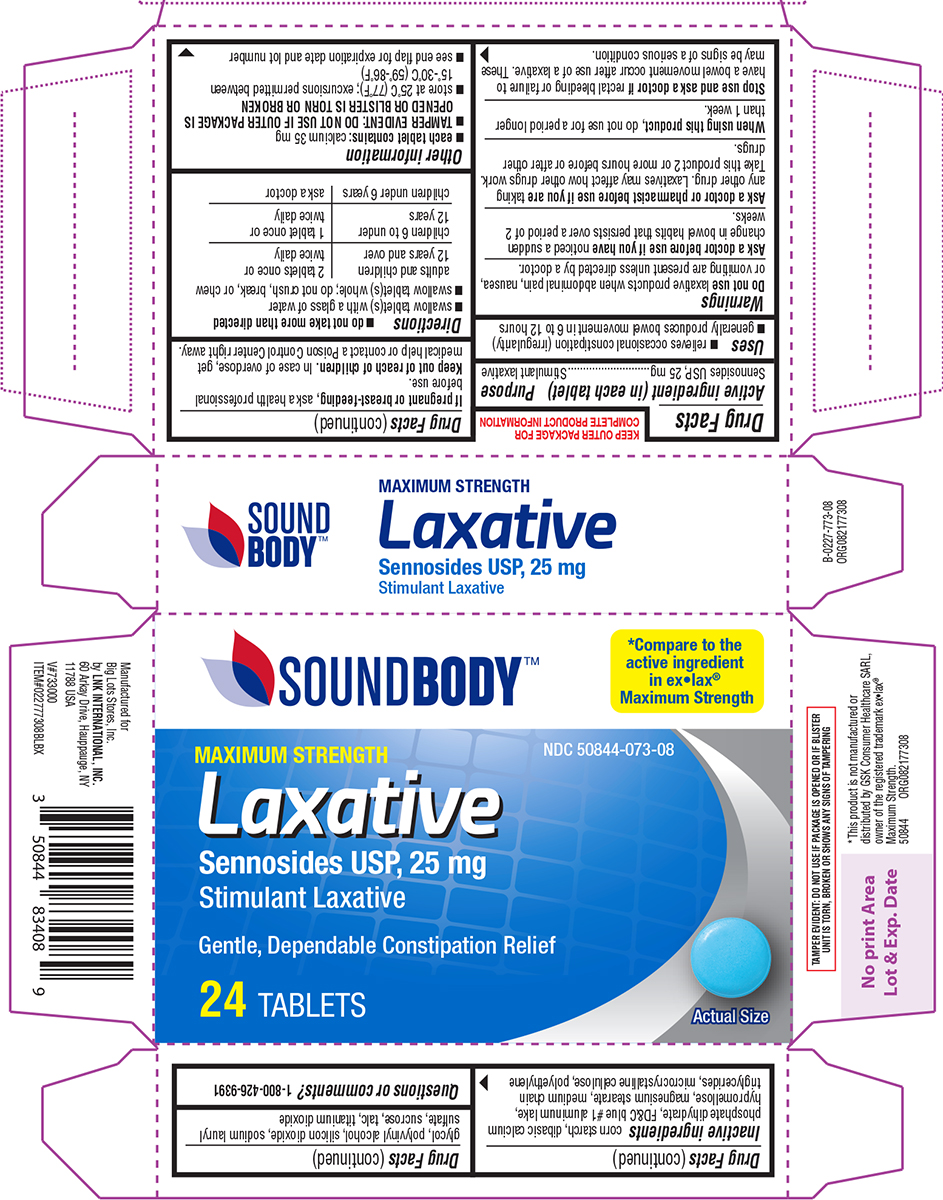
Label: LAXATIVE- sennosides tablet, film coated
- NDC Code(s): 50844-073-08
- Packager: L.N.K. International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor
Ask a doctor before use if you have
noticed a sudden change in bowel habits that persists over a period of 2 weeks.
Ask a doctor or pharmacist before use if you are
taking any other drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after other drugs.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
SOUNDBODY™
*Compare to the
active ingredient
in ex•lax®
Maximum StrengthNDC 50844-073-08
MAXIMUM STRENGTH
Laxative
Sennosides USP, 25 mg
Stimulant LaxativeGentle, Dependable Constipation Relief
24 TABLETS
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING*This product is not manufactured or
distributed by GSK Consumer Healthcare SARL,
owner of the registered trademark ex•lax®
Maximum Strength.
50844 ORG082177308Manufactured for
Big Lots Stores, Inc.
by LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY
11788 USA
V#733000
ITEM#022777308BLBX
Sound Body 44-773
-
INGREDIENTS AND APPEARANCE
LAXATIVE
sennosides tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50844-073 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 25 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape ROUND Size 10mm Flavor Imprint Code L7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50844-073-08 2 in 1 CARTON 07/05/2022 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/05/2022 Labeler - L.N.K. International, Inc. (038154464) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(50844-073) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(50844-073) , pack(50844-073) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(50844-073) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(50844-073) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(50844-073)