Label: NEOMYCIN AND POLYMYXIN B SULFATES AND HYDROCORTISONE- neomycin sulfate, polymyxin b sulfate and hydrocortisone suspension/ drops
- NDC Code(s): 53002-9000-1
- Packager: RPK Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 24208-635
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx only
-
DESCRIPTION
Neomycin and polymyxin B sulfates and hydrocortisone otic suspension, USP is a sterile antibacterial and anti-inflammatory suspension for otic use.
Each mL contains:
Actives:neomycin sulfate equivalent to 3.5 mg neomycin base, polymyxin B sulfate equivalent to 10,000 polymyxin B units, and hydrocortisone 10 mg (1%); Inactives:cetyl alcohol (0.9%), polysorbate 80, propylene glycol, purified water. Sulfuric acid may be added to adjust pH (3.0 - 7.0).
Preservative:thimerosal 0.01%.
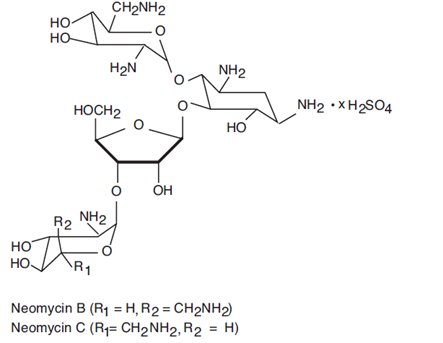
Neomycin sulfate is the sulfate salt of neomycin B and C, which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae). It has a potency equivalent of not less than 600 mcg of neomycin standard per mg, calculated on an anhydrous basis. The structural formulae are:
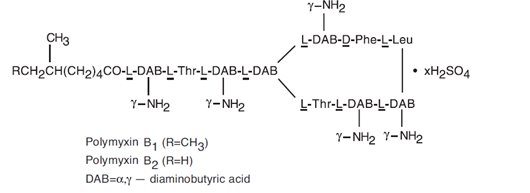
Polymyxin B sulfate is the sulfate salt of polymyxin B1 and B2, which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per mg, calculated on an anhydrous basis. The structural formulae are:
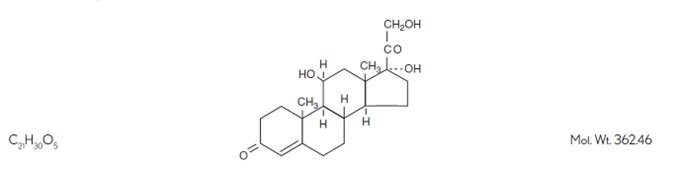
Hydrocortisone, 11β, 17, 21-trihydroxypregn-4-ene-3, 20-dione, is an anti-inflammatory hormone. Its structural formula is:
-
CLINICAL PHARMACOLOGY
Corticoids suppress the inflammatory response to a variety of agents and they may delay healing. Since corticoids may inhibit the body's defense mechanism against infection, a concomitant antimicrobial drug may be used when this inhibition is considered to be clinically significant in a particular case.
The anti-infective components in the combination are included to provide action against specific organisms susceptible to them. Neomycin sulfate and polymyxin B sulfate together are considered active against the following microorganisms: Staphylococcus aureus, Escherichia coli, Haemophilus influenzae, Klebsiella-Enterobacter species, Neisseria species, and Pseudomonas aeruginosa. This product does not provide adequate coverage against Serratia marcescens and streptococci, including Streptococcus pneumoniae.
The relative potency of corticosteroids depends on the molecular structure, concentration, and release from the vehicle.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Neomycin can induce permanent sensorineural hearing loss due to cochlear damage, mainly destruction of hair cells in the organ of Corti. The risk is greater with prolonged use. Therapy should be limited to 10 consecutive days (see PRECAUTIONS-General). Patients being treated with eardrops containing neomycin should be under close clinical observation. Neomycin and polymyxin B sulfates and hydrocortisone otic suspension should not be used in any patient with a perforated tympanic membrane.
Discontinue promptly if sensitization or irritation occurs.
Neomycin sulfate may cause cutaneous sensitization. A precise incidence of hypersensitivity reactions (primarily skin rash) due to topical neomycin is not known.
When using neomycin-containing products to control secondary infection in the chronic dermatoses, such as chronic otitis externa or stasis dermatitis, it should be borne in mind that the skin in these conditions is more liable than is normal skin to become sensitized to many substances, including neomycin. The manifestation of sensitization to neomycin is usually a low-grade reddening with swelling, dry scaling, and itching; it may be manifest simply as a failure to heal. Periodic examination for such signs is advisable, and the patient should be told to discontinue the product if they are observed. These symptoms regress quickly on withdrawing the medication. Neomycin containing applications should be avoided for the patient thereafter.
-
PRECAUTIONS
General: As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi.
If the infection is not improved after 1 week, cultures and susceptibility tests should be repeated to verify the identity of the organism and to determine whether therapy should be changed.
Treatment should not be continued for longer than 10 days.
Allergic cross-reactions may occur which could prevent the use of any or all of the following antibiotics for the treatment of future infections: kanamycin, paromomycin, streptomycin, and possibly gentamicin.
Information for Patients: Avoid contaminating the bottle tip with material from the ear, fingers, or other source. This caution is necessary if the sterility of the drops is to be preserved.
If sensitization or irritation occurs, discontinue use immediately and contact your physician.
Do not use in the eyes.
SHAKE WELL BEFORE USING.
Laboratory Tests: Systemic effects of excessive levels of hydrocortisone may include a reduction in the number of circulating eosinophils and a decrease in urinary excretion of 17-hydroxycorticosteroids.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies in animals (rats, rabbits, mice) showed no evidence of carcinogenicity attributable to oral administration of corticosteroids.
Pregnancy
Teratogenic Effects: Corticosteroids have been shown to be teratogenic in rabbits when applied topically at concentrations of 0.5% on days 6 to 18 of gestation and in mice when applied topically at a concentration of 15% on days 10 to 13 of gestation. There are no adequate and well-controlled studies in pregnant women. Corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: Hydrocortisone appears in human milk following oral administration of the drug. Since systemic absorption of hydrocortisone may occur when applied topically, caution should be exercised when neomycin and polymyxin B sulfates and hydrocortisone otic suspension is used by a nursing woman.
Pediatric Use: The safety and effectiveness of neomycin and polymyxin B sulfates and hydrocortisone otic suspension in otitis externa have been established in the pediatric age group.
Geriatric Use: Clinical studies of neomycin and polymyxin B sulfates and hydrocortisone otic suspension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
ADVERSE REACTIONS
Neomycin occasionally causes skin sensitization. Ototoxicity and nephrotoxicity have also been reported (see WARNINGS). Adverse reactions have occurred with topical use of antibiotic combinations including neomycin and polymyxin B. Exact incidence figures are not available since no denominator of treated patients is available. The reaction occurring most often is allergic sensitization. In one clinical study, using a 20% neomycin patch, neomycin-induced allergic skin reactions occurred in two of 2,175 (0.09%) individuals in the general population. In another study, the incidence was found to be approximately 1%.
The following local adverse reactions have been reported with topical corticosteroids, especially under occlusive dressings: burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae and miliaria. Stinging and burning have been reported rarely when this drug has gained access to the middle ear.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
Therapy with this product should be limited to 10 consecutive days. The external auditory canal should be thoroughly cleansed and dried with a sterile cotton applicator.
For adults, 4 drops of the suspension should be instilled into the affected ear 3 to 4 times daily.
For children, 3 drops are suggested because of the smaller capacity of the ear canal.
The patient should lie with the affected ear upward and then the drops should be instilled. This position should be maintained for 5 minutes to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear.
If preferred, a cotton wick may be inserted into the canal and then the cotton may be saturated with the suspension. This wick should be kept moist by adding further suspension every 4 hours. The wick should be replaced at least once every 24 hours.
SHAKE WELL BEFORE USING.
- HOW SUPPLIED
- Neo Poly HC Otic Suspension
-
INGREDIENTS AND APPEARANCE
NEOMYCIN AND POLYMYXIN B SULFATES AND HYDROCORTISONE
neomycin sulfate, polymyxin b sulfate and hydrocortisone suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53002-9000(NDC:24208-635) Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 mL NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 mL POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SULFURIC ACID (UNII: O40UQP6WCF) THIMEROSAL (UNII: 2225PI3MOV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53002-9000-1 1 in 1 CARTON 10/01/2017 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064065 08/28/1996 Labeler - RPK Pharmaceuticals, Inc. (147096275) Establishment Name Address ID/FEI Business Operations RPK Pharmaceuticals, Inc. 147096275 RELABEL(53002-9000)