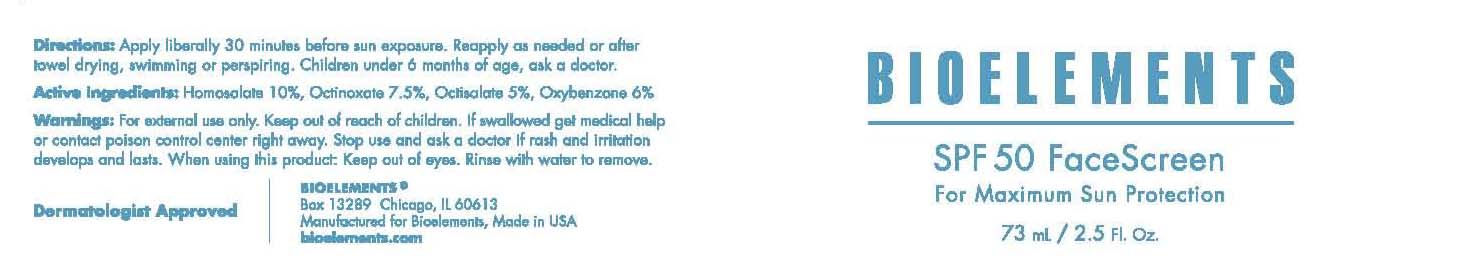
Label: BIOELEMENTS, INC. SPF 50 FACESCREEN- homosalate, octinoxate, octisalate, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 49825-117-01, 49825-117-02 - Packager: Bioelements, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 17, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients Purpose
Homosalate 10% Sunscreen
Octinoxate 7.5% Sunscreen
Octisalate 5% Sunscreen
Oxybenzone 6% Sunscreen
Warnings
For external use only.
Keep out of reach of children. If swallowed get medical help or contact poison control center right away.
Stop use and ask a doctor if rash and irritation develops and lasts
When using this product: keep out of eyes. Rinse with water to remove.
Uses:
for skin highly sensitive to sunburn
Directions:
Apply liberally 30 minutes before sun exposure. Reapply as needed or after towel drying, swimming or perspiring. Children under 6 months of age, ask a doctor.
Water (Aqua) (Eau), Aloe Barbadensis Leaf Juice, Glycerine, Cetyl Alcohol, Stearic Acid, C12-15 Alkyl Benzoate, Retinyl Palmitate, Tocopheryl Acetate, Panax Ginseng (Ginseng) Root Extract, Centella Asiatica (Gatu Kola) Extract, Angelica Polymorpha Sinensis (Dong Quai) Root Extract, Nasturtium Officinale (Watercress) Extract, Rhus Glabra (Sumac) Extract, Chamomilla Recutita (Matricaria) Flower Extract, Dimethicone, Dea-cetyl Phosphate, VP/Eicosene Copolymer, Phenoxyethanol, Butylene Glycol, Triethanolamine, Carbomer, Titanium Dioxide, Glycereth-2 Cocoate, Iodopropynyl Butylcarbamate

- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOELEMENTS, INC. SPF 50 FACESCREEN
homosalate, octinoxate, octisalate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49825-117 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 mL in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mL in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mL in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE (UNII: V5VD430YW9) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) STEARIC ACID (UNII: 4ELV7Z65AP) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ASIAN GINSENG (UNII: CUQ3A77YXI) CENTELLA ASIATICA (UNII: 7M867G6T1U) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) RORIPPA NASTURTIUM-AQUATICUM (UNII: YH89GMV676) RHUS GLABRA BARK (UNII: 7XC0E9WP6U) CHAMOMILE (UNII: FGL3685T2X) DIMETHICONE (UNII: 92RU3N3Y1O) DIETHANOLAMINE CETYL PHOSPHATE (UNII: 4UG0316V9S) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER 934 (UNII: Z135WT9208) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERETH-2 COCOATE (UNII: JWM00VS7HC) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49825-117-02 1 in 1 BOX 1 NDC:49825-117-01 73 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/01/2010 Labeler - Bioelements, Inc. (174813923) Registrant - Bioelements, Inc. (174813923) Establishment Name Address ID/FEI Business Operations Bioelements, Inc. 174813923 manufacture