Label: 24HR HEALTH JOINT- vitamin spray
- NHRIC Code(s): 80893-008-15
- Packager: USA Health
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated November 6, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Precaution
- Warnings
- Safe Handling
- Dosage
- Supplement Facts
- PRINCIPAL DISPLAY PANEL
-
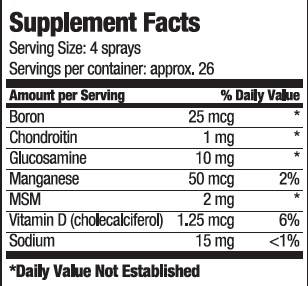
INGREDIENTS AND APPEARANCE
24HR HEALTH JOINT
vitamin sprayProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:80893-008 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BORON (UNII: N9E3X5056Q) (BORON - UNII:N9E3X5056Q) BORON 25 ug in 15 mL CHONDROITIN SULFATE SODIUM (BOVINE) (UNII: 8QTV3DTT8W) (CHONDROITIN SULFATE (BOVINE) - UNII:6IC1M3OG5Z) CHONDROITIN SULFATE (BOVINE) 1 mg in 15 mL GLUCOSAMINE (UNII: N08U5BOQ1K) (GLUCOSAMINE - UNII:N08U5BOQ1K) GLUCOSAMINE 10 mg in 15 mL MANGANESE (UNII: 42Z2K6ZL8P) (MANGANESE - UNII:42Z2K6ZL8P) MANGANESE 50 ug in 15 mL VITAMIN D (UNII: 9VU1KI44GP) (CHOLECALCIFEROL - UNII:1C6V77QF41) VITAMIN D 1.25 ug in 15 mL SODIUM (UNII: 9NEZ333N27) (SODIUM - UNII:9NEZ333N27) SODIUM 15 mg in 15 mL DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) (DIMETHYL SULFONE - UNII:9H4PO4Z4FT) DIMETHYL SULFONE 2 mg in 15 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sucralose (UNII: 96K6UQ3ZD4) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:80893-008-15 15 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 11/01/2020 Labeler - USA Health (117624854) Registrant - USA Health (117624854) Establishment Name Address ID/FEI Business Operations Streamline Manufacturing 098915617 manufacture