Label: CONCEPTROL OPTIONS- nonoxynol gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 34362-0300-1, 34362-0300-5 - Packager: Caldwell Consumer Health LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 18, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
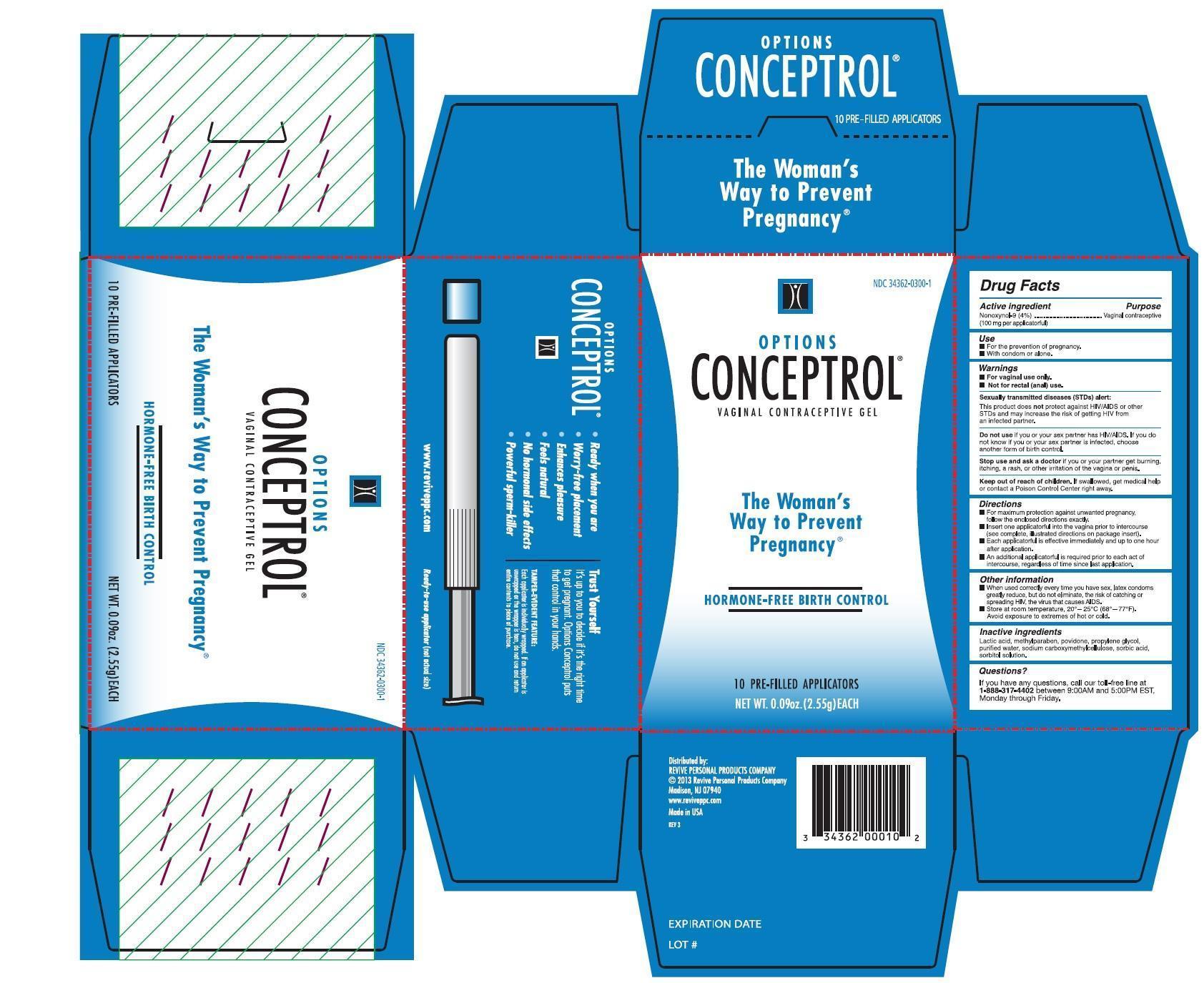
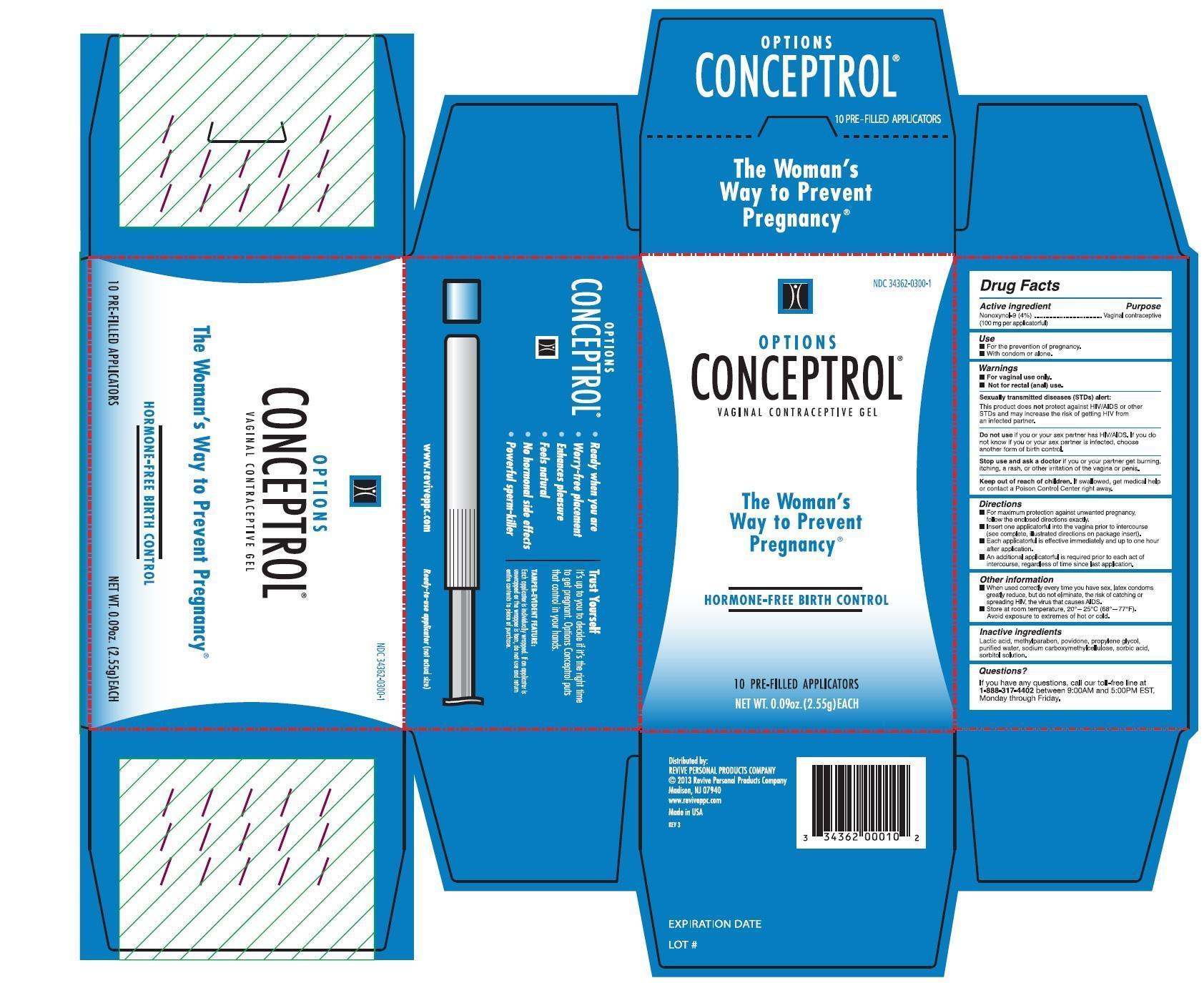
- Active ingredient
- Uses
-
WARNINGS
For vaginal use only.
Not for rectal (anal) use.
Sexually transmitted diseases (STDs) alert:
This product does not protect against HIV/AIDS or other STDs and may increase the risk of getting HIV from an infected partner.
Do not use if you or your sex partner has HIV/AIDS. If you do not know if your sex partner is infected, choose another form of birth control.
Stop use and ask a doctor if you or your partner gets burning, itching, a rash, or other irritation or the vagina or penis.
- Directions
- DOSAGE & ADMINISTRATION
- Other Information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CONCEPTROL OPTIONS
nonoxynol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:34362-0300 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NONOXYNOL-9 (UNII: 48Q180SH9T) (NONOXYNOL-9 - UNII:48Q180SH9T) NONOXYNOL-9 4.0 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SORBIC ACID (UNII: X045WJ989B) METHYLPARABEN (UNII: A2I8C7HI9T) LACTIC ACID (UNII: 33X04XA5AT) POVIDONE K29/32 (UNII: 390RMW2PEQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:34362-0300-5 10 in 1 CARTON 1 NDC:34362-0300-1 2.55 g in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/01/2011 Labeler - Caldwell Consumer Health LLC (828558713) Registrant - Lornamead (126440440) Establishment Name Address ID/FEI Business Operations DPT Laboratories 832224526 manufacture(34362-0300) , pack(34362-0300)