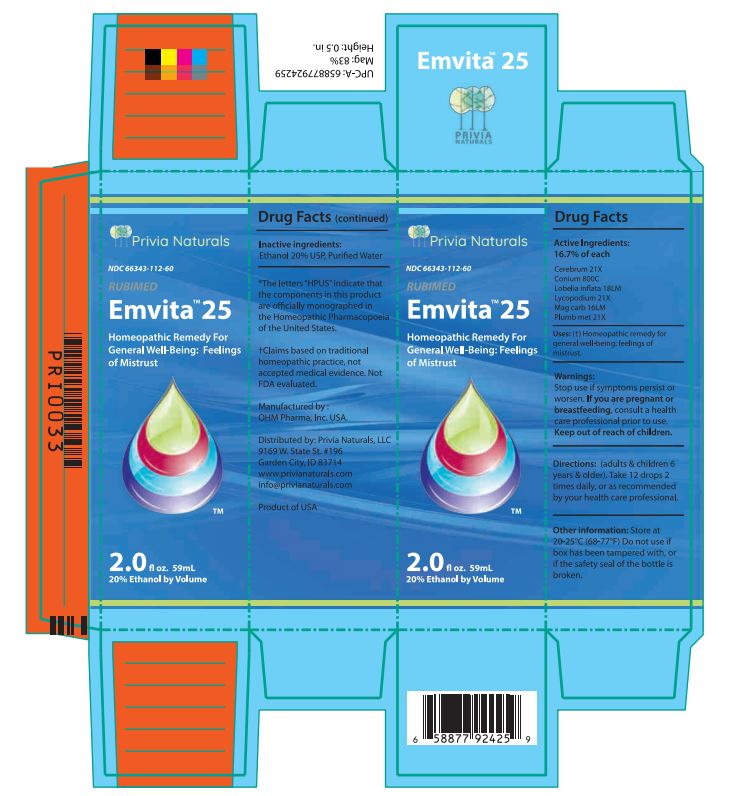
Label: EMVITA 25- cerebrum, conium, lobelia inflata, lycopodium, mag carb, plumb met liquid
- NDC Code(s): 66343-112-60
- Packager: RUBIMED AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Drug Facts Active Ingredients: (HPUS*) 16.7% of each
Cerebrum 21X Conium 800C
Lobelia inflata 18LM Lycopodium 21X
Mag carb 16LM Plumb met 21X*The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States. †Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EMVITA 25
cerebrum, conium, lobelia inflata, lycopodium, mag carb, plumb met liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66343-112 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUS SCROFA CEREBRUM (UNII: 4GB5DQR532) (SUS SCROFA CEREBRUM - UNII:4GB5DQR532) SUS SCROFA CEREBRUM 21 [hp_X] in 60 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 800 [hp_C] in 60 mL LOBELIA INFLATA WHOLE (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA WHOLE 18 [hp_M] in 60 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 21 [hp_X] in 60 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 16 [hp_M] in 60 mL LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 21 [hp_X] in 60 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66343-112-60 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/23/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/23/2022 Labeler - RUBIMED AG (480582035)