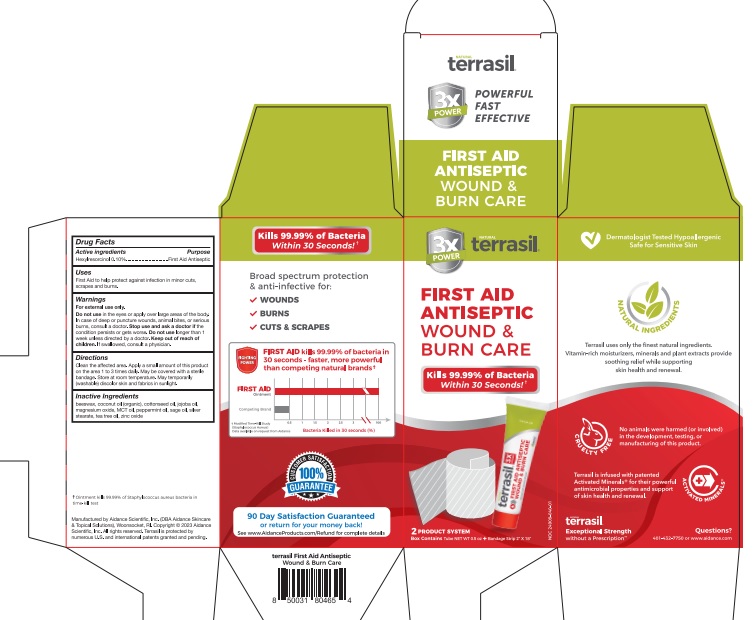
Label: TERRASIL FIRST AID ANTISEPTIC WOUND AND BURN CARE KIT- hexylresorcinol kit
- NDC Code(s): 24909-040-01, 24909-041-14
- Packager: Aidance Scientific, Inc, DBA Aidance Skincare & Topical Solutions
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient Purpose
- PURPOSE
- Uses
-
Warnings
For external use only.
Do not usein the eyes or apply over large areas of the body. In case of deep or puncture wounds, animal bites, or serious burns, consult a doctor.
Stop use and ask a doctor if the condition persists or gets worse. Do not uselonger than 1 week unless directed by a doctor. Keep out of reach of children. If swallowed, consult a physician.
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive Ingredients
- Other information
- Product label
-
INGREDIENTS AND APPEARANCE
TERRASIL FIRST AID ANTISEPTIC WOUND AND BURN CARE KIT
hexylresorcinol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24909-040 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24909-040-01 1 in 1 KIT 10/02/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 14 g Part 2 1 Part 1 of 2 TERRASIL FIRST AID ANTISEPTIC WOUND AND BURN CARE
hexylresorcinol ointmentProduct Information Item Code (Source) NDC:24909-041 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEXYLRESORCINOL (UNII: R9QTB5E82N) (HEXYLRESORCINOL - UNII:R9QTB5E82N) HEXYLRESORCINOL 0.1 g in 100 g Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) COCONUT OIL (UNII: Q9L0O73W7L) COTTONSEED OIL (UNII: H3E878020N) JOJOBA OIL (UNII: 724GKU717M) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) PALM OIL (UNII: 5QUO05548Z) PEPPERMINT OIL (UNII: AV092KU4JH) SAGE OIL (UNII: U27K0H1H2O) SILVER STEARATE (UNII: 4H6PCL92ZN) TEA TREE OIL (UNII: VIF565UC2G) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24909-041-14 14 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 Part 2 of 2 TERRASIL HOSPITAL GRADE BANDAGING
strip, adhesive, closure, skinProduct Information Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Exempt device ABC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/02/2023 Labeler - Aidance Scientific, Inc, DBA Aidance Skincare & Topical Solutions (018950611) Establishment Name Address ID/FEI Business Operations Aidance Scientific, Inc, DBA Aidance Skincare & Topical Solutions 018950611 manufacture(24909-040, 24909-041)