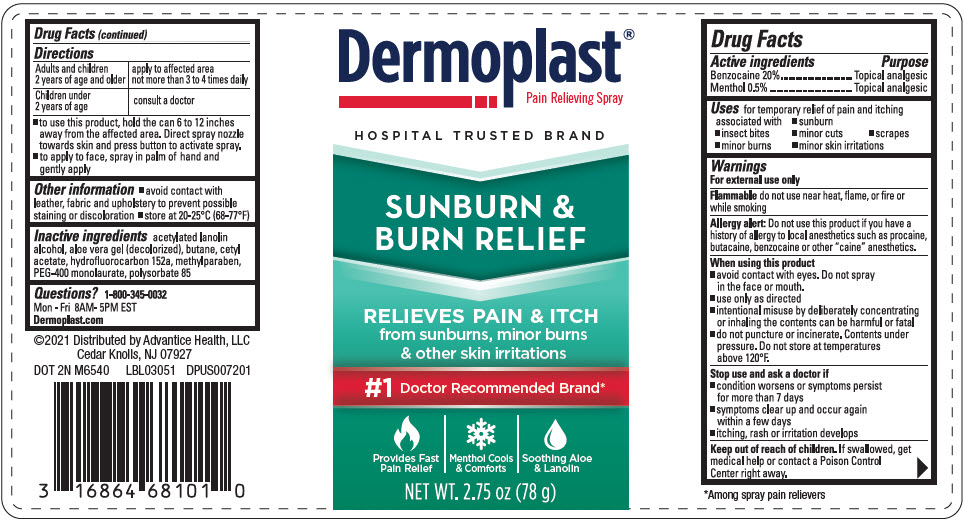
Label: DERMOPLAST SUNBURN AND BURN RELIEF- benzocaine and levomenthol spray
- NDC Code(s): 16864-681-01
- Packager: Advantice Health, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 24, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
Flammable do not use near heat, flame, or fire or while smoking
Allergy alert
Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
When using this product
- avoid contact with eyes. Do not spray in the face or mouth.
- use only as directed
- intentional misuse by deliberately concentrating or inhaling the contents can be harmful or fatal
- do not puncture or incinerate. Contents under pressure. Do not store at temperatures above 120°F.
-
Directions
Adults and children 2 years of age and older apply to affected area not more than 3 to 4 times daily Children under 2 years of age consult a doctor - to use this product, hold the can 6 to 12 inches away from the affected area. Direct spray nozzle towards skin and press button to activate spray.
- to apply to face, spray in palm of hand and gently apply
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 78 g Can Label
-
INGREDIENTS AND APPEARANCE
DERMOPLAST SUNBURN AND BURN RELIEF
benzocaine and levomenthol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16864-681 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 g LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 5 mg in 1 g Inactive Ingredients Ingredient Name Strength PEG-8 LAURATE (UNII: 762O8IWA10) POLYSORBATE 85 (UNII: A7F3N56197) METHYLPARABEN (UNII: A2I8C7HI9T) ALOE VERA LEAF (UNII: ZY81Z83H0X) ACETYLATED LANOLIN ALCOHOLS (UNII: SNN716810P) CETYL ACETATE (UNII: 4Q43814HXS) BUTANE (UNII: 6LV4FOR43R) 1,1-DIFLUOROETHANE (UNII: 0B1U8K2ME0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16864-681-01 78 g in 1 CAN; Type 0: Not a Combination Product 05/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 05/15/2021 Labeler - Advantice Health, LLC (192527062)