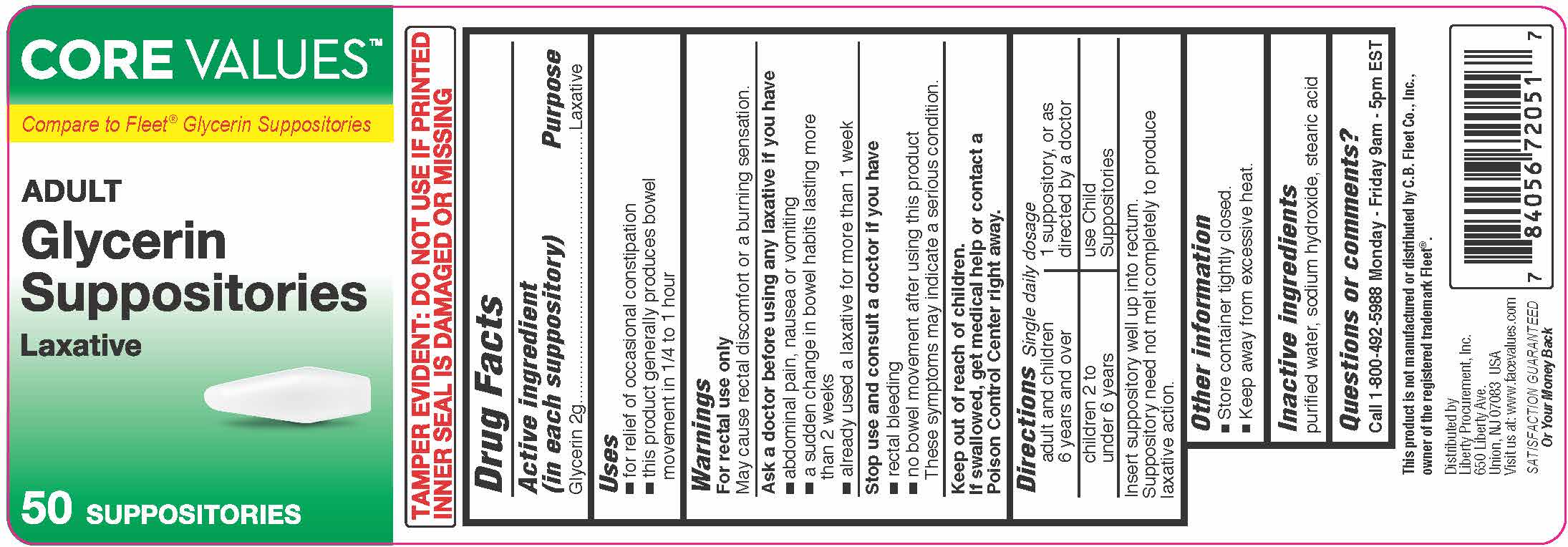
Label: GLYCERIN suppository
- NDC Code(s): 52316-720-51
- Packager: DSC Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each suppository)
- Purpose
- Uses
-
Warnings
For rectal use only
May cause rectal discomfort or a burning sensation.
Ask a doctor before using any laxative if you have
- abdominal pain, nausea or vomiting
- a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative formore than 1 week
- Directions-single daily dosage
- Other information
- Inactive ingredients
- Questions or comments?
- Label
-
INGREDIENTS AND APPEARANCE
GLYCERIN
glycerin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52316-720 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52316-720-51 50 in 1 JAR; Type 0: Not a Combination Product 08/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 08/01/2017 Labeler - DSC Laboratories Inc. (097807374) Registrant - DSC Laboratories Inc. (097807374) Establishment Name Address ID/FEI Business Operations DSC Laboratories Inc. 097807374 manufacture(52316-720)