Label: HIMS- minoxidil 5% liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 71730-042-08 - Packager: Clubroom, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 30, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
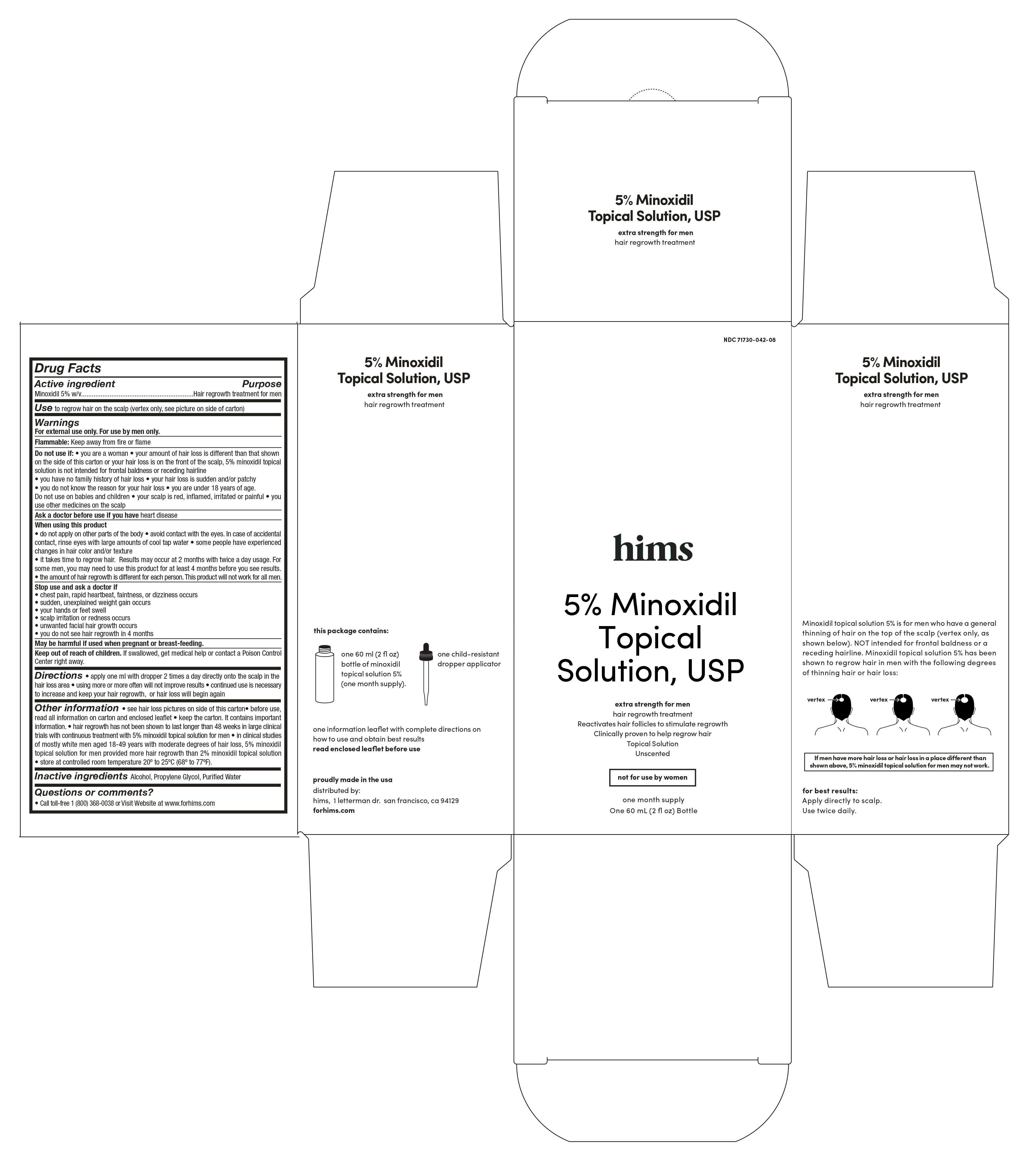
For external use only
For use by men only
Flammable: Keep away from fire or flame
Do not use if
•you are a woman
•your amount of hair loss is different than that shown on the side of this carton or your hair loss is on the front of the scalp. Minoxidil Topical Solution, 5% is not intended for frontal baldness or receding hairline.
•you have no family history of hair loss
•your hair loss is sudden and/or patchy
•you do not know the reason for your hair loss
•you are under 18 years of age. Do not use on babies and children.
•your scalp is red, inflamed, infected, irritated, or painful
•you use other medicines on the scalp
Ask a doctor before use if you have heart disease
When using this product•do not apply on other parts of the body
•avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
•some people have experienced changes in hair color and/or texture
•it takes time to regrow hair. Results may occur at 2 months with twice a day usage. For some men, you may need to use this product for at least 4 months before you see results.
•the amount of hair regrowth is different for each person. This product will not work for all men.
Stop use and ask a doctor if•chest pain, rapid heartbeat, faintness, or dizziness occurs
•sudden, unexplained weight gain occurs
•your hands or feet swell
•scalp irritation or redness occurs
•unwanted facial hair growth occurs
•you do not see hair regrowth in 4 months
May be harmful if used when pregnant or breast-feeding
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
OTHER SAFETY INFORMATION
• see hair loss pictures on side of this carton
• before use, read all information on carton and enclosed leaflet
• keep the carton. It contains important information.
• hair regrowth has not been shown to last longer than 48 weeks in large clinical trials with continuous treatment with 5% minoxidil topical solution for men
• in clinical studies of mostly white men aged 18-49 years with moderate degrees of hair loss, 5% minoxidil topical solution for men provided more hair regrowth than 2% minoxidil topical solution
• store at controlled room temperature 20º to 25ºC (68º to 77ºF). - INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HIMS
minoxidil 5% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71730-042 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71730-042-08 1 in 1 BOX 11/18/2017 1 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075518 11/01/2017 Labeler - Clubroom, Inc. (080696472)