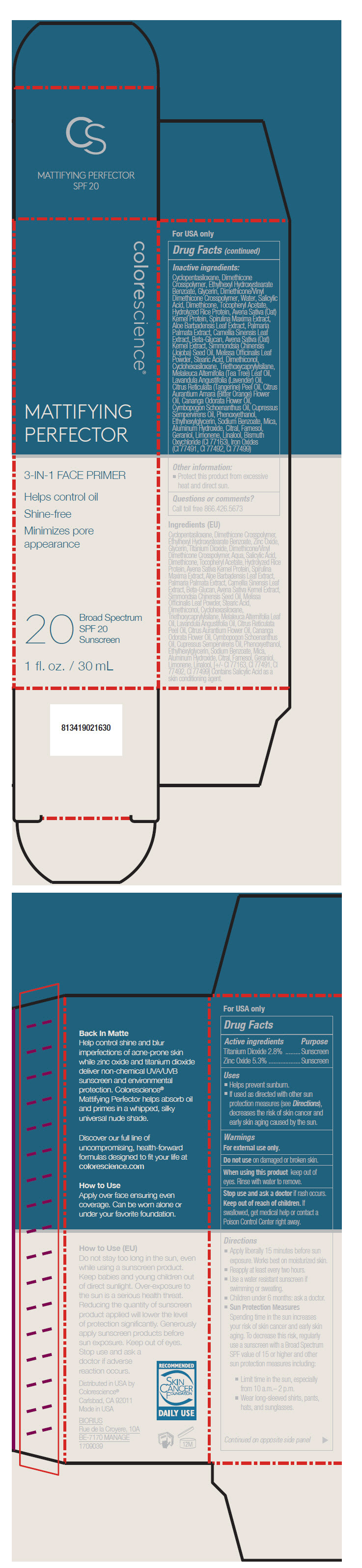
Label: MATTIFYING PERFECTOR SPF 20 SUNSCREEN- titanium dioxide and zinc oxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 68078-018-19 - Packager: Colorescience
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure. Works best on moisturized skin.
- Reapply at least every two hours.
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months: ask a doctor.
-
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:- Limit time in the sun, especially from 10 a.m.– 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
-
Inactive ingredients
Cyclopentasiloxane, Dimethicone Crosspolymer, Ethylhexyl Hydroxystearate Benzoate, Glycerin, Dimethicone/Vinyl Dimethicone Crosspolymer, Water, Salicylic Acid, Dimethicone, Tocopheryl Acetate, Hydrolyzed Rice Protein, Avena Sativa (Oat) Kernel Protein, Spirulina Maxima Extract, Aloe Barbadensis Leaf Extract, Palmaria Palmata Extract, Camellia Sinensis Leaf Extract, Beta-Glucan, Avena Sativa (Oat) Kernel Extract, Simmondsia Chinensis (Jojoba) Seed Oil, Melissa Officinalis Leaf Powder, Stearic Acid, Dimethiconol, Cyclohexasiloxane, Triethoxycaprylylsilane, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Lavandula Angustifolia (Lavender) Oil, Citrus Reticulata (Tangerine) Peel Oil, Citrus Aurantium Amara (Bitter Orange) Flower Oil, Cananga Odorata Flower Oil, Cymbopogon Schoenanthus Oil, Cupressus Sempervirens Oil, Phenoxyethanol, Ethylhexylglycerin, Sodium Benzoate, Mica, Aluminum Hydroxide, Citral, Farnesol, Geraniol, Limonene, Linalool, Bismuth Oxychloride (CI 77163), Iron Oxides (CI 77491, CI 77492, CI 77499)
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
MATTIFYING PERFECTOR SPF 20 SUNSCREEN
titanium dioxide and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68078-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 28 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 53 mg in 1 mL Inactive Ingredients Ingredient Name Strength Cyclomethicone 5 (UNII: 0THT5PCI0R) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) Ethylhexyl Hydroxystearate Benzoate (UNII: 3W8F25684B) Glycerin (UNII: PDC6A3C0OX) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) Water (UNII: 059QF0KO0R) Salicylic Acid (UNII: O414PZ4LPZ) Dimethicone (UNII: 92RU3N3Y1O) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) RICE BRAN (UNII: R60QEP13IC) Oat (UNII: Z6J799EAJK) ARTHROSPIRA MAXIMA (UNII: 9K7IG15M0Q) ALOE VERA LEAF (UNII: ZY81Z83H0X) DULSE (UNII: 7832HOY4ZQ) GREEN TEA LEAF (UNII: W2ZU1RY8B0) POLYSACCHARIDE-K (UNII: 3X48A86C8K) JOJOBA OIL (UNII: 724GKU717M) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) Stearic Acid (UNII: 4ELV7Z65AP) DIMETHICONOL (100000 CST) (UNII: OSA9UP217S) CYCLOMETHICONE 6 (UNII: XHK3U310BA) Triethoxycaprylylsilane (UNII: LDC331P08E) TEA TREE OIL (UNII: VIF565UC2G) LAVENDER OIL (UNII: ZBP1YXW0H8) MANDARIN OIL (UNII: NJO720F72R) CITRUS AURANTIUM FLOWER OIL (UNII: D4BGE91OXH) CANANGA OIL (UNII: 8YOY78GNNX) Cymbopogon Schoenanthus Oil (UNII: XE7K568ILO) CUPRESSUS SEMPERVIRENS LEAF OIL (UNII: M7QUY89S4O) Phenoxyethanol (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Sodium Benzoate (UNII: OJ245FE5EU) Mica (UNII: V8A1AW0880) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Citral (UNII: T7EU0O9VPP) Farnesol (UNII: EB41QIU6JL) Geraniol (UNII: L837108USY) Limonene, (+)- (UNII: GFD7C86Q1W) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68078-018-19 1 in 1 CARTON 02/01/2017 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/01/2017 Labeler - Colorescience (128731929)