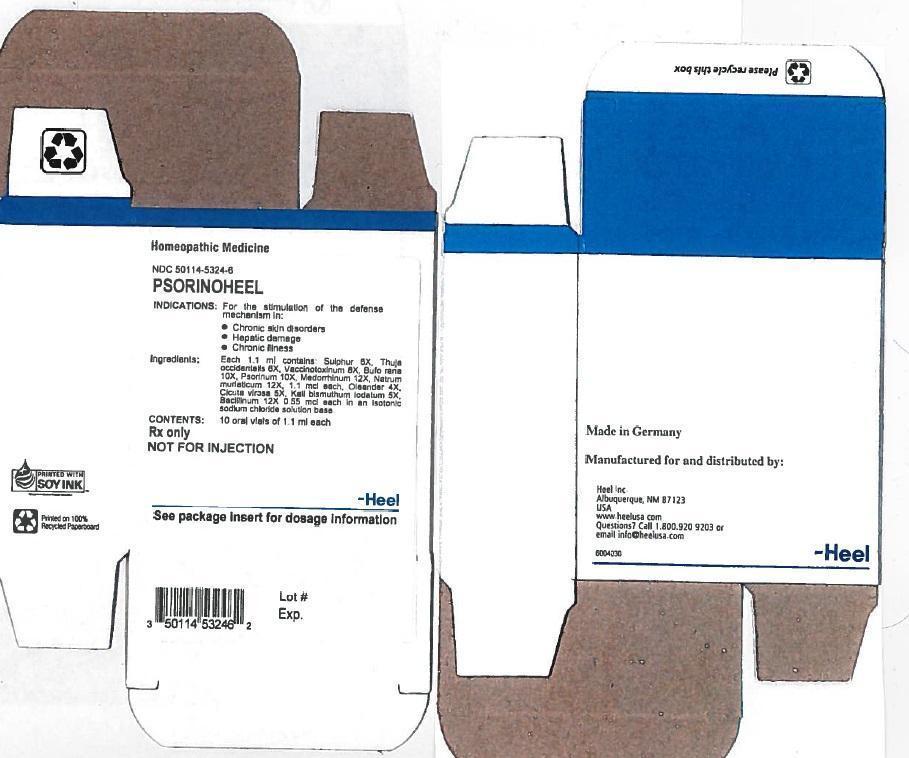
Label: PSORINOHEEL- sulfur, thuja occidentalis leafy twig, vaccinia virus strain new york city board, gonorrheal urethral secretion human,sodium chloride, nerium oleander leaf,cicuta virosa root, solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 50114-5324-6 - Packager: Heel Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 19, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each1.1 ml ampule contains:
Ingredient Name
Potency
Quantity
Bacillinum
12x
0.55µ
Bufo rana
10x
1.1µ
Cictuta virosa
5x
0.55µ
Kali bismuthum iodatum
5x
0.55 µ
Medorrhinum
12x
1.1µ
Natrum muriaticum
12x
1.1µ
Oleander
4x
0.55µ
Psorinum
10x
1.1µ
Sulphur
6x
1.1µ
Thuja occidentalis
6x
1.1µ
Vaccinotoxinum
8x
1.1µ
Inactive Ingredient: Isotonic Sodium Chloride solution - INDICATION AND USAGE
- DOSAGE AND ADMINISTRATION
- CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- ADVERSE REACTIONS
- OVERDOSAGE
- CLINICAL PHARMACOLOGY
- DOSAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PSORINOHEEL
sulfur, thuja occidentalis leafy twig, vaccinia virus strain new york city board, gonorrheal urethral secretion human,sodium chloride, nerium oleander leaf,cicuta virosa root, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50114-5324 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_X] in 1.1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_X] in 1.1 mL VACCINIA VIRUS STRAIN NEW YORK CITY BOARD OF HEALTH LIVE ANTIGEN (UNII: 4SV59689SK) (VACCINIA VIRUS STRAIN NEW YORK CITY BOARD OF HEALTH LIVE ANTIGEN - UNII:4SV59689SK) VACCINIA VIRUS STRAIN NEW YORK CITY BOARD OF HEALTH LIVE ANTIGEN 8 [hp_X] in 1.1 mL BUFO BUFO CUTANEOUS GLAND (UNII: Q59QU6N72Q) (BUFO BUFO CUTANEOUS GLAND - UNII:Q59QU6N72Q) BUFO BUFO CUTANEOUS GLAND 10 [hp_X] in 1.1 mL SCABIES LESION LYSATE (HUMAN) (UNII: 5UAU16Z1U4) (SCABIES LESION LYSATE (HUMAN) - UNII:5UAU16Z1U4) SCABIES LESION LYSATE (HUMAN) 10 [hp_X] in 1.1 mL GONORRHEAL URETHRAL SECRETION HUMAN (UNII: 9BZG9E3I8F) (GONORRHEAL URETHRAL SECRETION HUMAN - UNII:9BZG9E3I8F) GONORRHEAL URETHRAL SECRETION HUMAN 12 [hp_X] in 1.1 mL NERIUM OLEANDER LEAF (UNII: 7KV510R6H6) (NERIUM OLEANDER LEAF - UNII:7KV510R6H6) NERIUM OLEANDER LEAF 4 [hp_X] in 1.1 mL CICUTA VIROSA ROOT (UNII: YEA9P21S8N) (CICUTA VIROSA ROOT - UNII:YEA9P21S8N) CICUTA VIROSA ROOT 5 [hp_X] in 1.1 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 5 [hp_X] in 1.1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50114-5324-6 10 in 1 CARTON 1 1.1 mL in 1 AMPULE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/31/1993 Labeler - Heel Inc (102783016)