Label: BEERX COLD SORE TREATMENT- glycerin gel
- NDC Code(s): 51672-5309-7
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
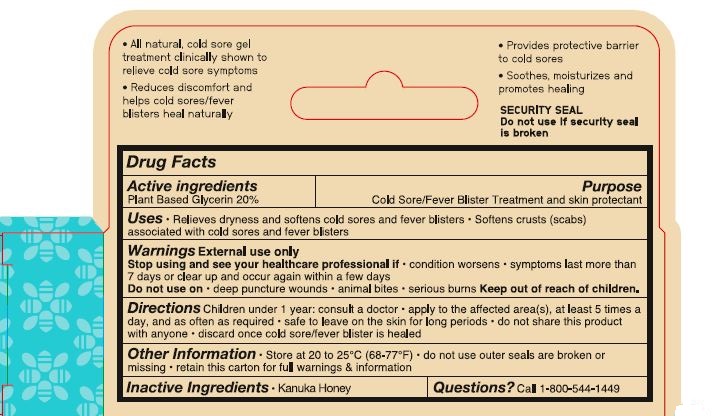
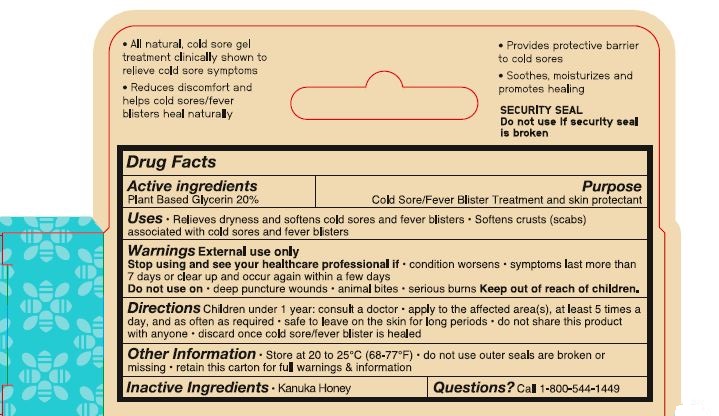
- ACTIVE INGREDIENT
- PURPOSE
- ACTIVE INGREDIENT
- Uses
- Warnings External use only
- Stop using and see your healthcare professional if
- Do not use on
- Keep out of reach of children.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- Other Information
- Inactive Ingredients
- Questions? Call 1-800-544-1449
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
beeRX™
NON-TOXIC
NOT TESTED
ON ANIMALS
COLORANT
AND FRAGRANCE
FREE
NATURALLY
SOURCED
Cold Sore Treatment
INGREDIENTS:
20% PLANT
BASED GLYCERIN
100%
NATURAL
.35 oz./
10 g
Clinically shown
to relieve cold
sore symptoms
and promote
healing
Country Of Origin New Zealand
INGREDIENTS Plant Based Glycerin,
Kanuka Honey
www.bee-rx.com
TARO
Dist. by Taro
Pharmaceuticals U.S.A. Inc.
Hawthorne, NY 10532
www.bee-rx.com
1-800-544-1449- All natural, cold sore gel
treatment clinically shown to
relieve cold sore symptoms
- Reduces discomfort and
help cold sores/fever
blisters heal naturally
- Provides protective barrier
to cold sores
- Soothes, moisturizes and
promotes healing
SECURITY SEAL
Do not use if security seal
is broken

beeRX™
Cold Sore
Treatment
INGREDIENTS:
20% PLANT
BASED GLYCERIN
100% NATURAL
.35 oz. / 10 g
MADE IN AUSTRALIA
INGREDIENTS Plant Based Glycerin, New Zealand Kanuka Honey
Retain packaging for full product uses, directions and warning. Tube
should be in a carton at the time of purchase. Store at 20-25C
(68-77F).
QUESTIONS?
Call 1-800-544-1449 or visit
www.bee-rx.com
TARO
Dist. by: Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
www.bee-rx.com
- All natural, cold sore gel
-
INGREDIENTS AND APPEARANCE
BEERX COLD SORE TREATMENT
glycerin gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51672-5309 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Glycerin (UNII: PDC6A3C0OX) (Glycerin - UNII:PDC6A3C0OX) Glycerin .2 g in 1 g Inactive Ingredients Ingredient Name Strength Honey (UNII: Y9H1V576FH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51672-5309-7 1 in 1 BOX 06/01/2023 1 10 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 06/01/2023 Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370)