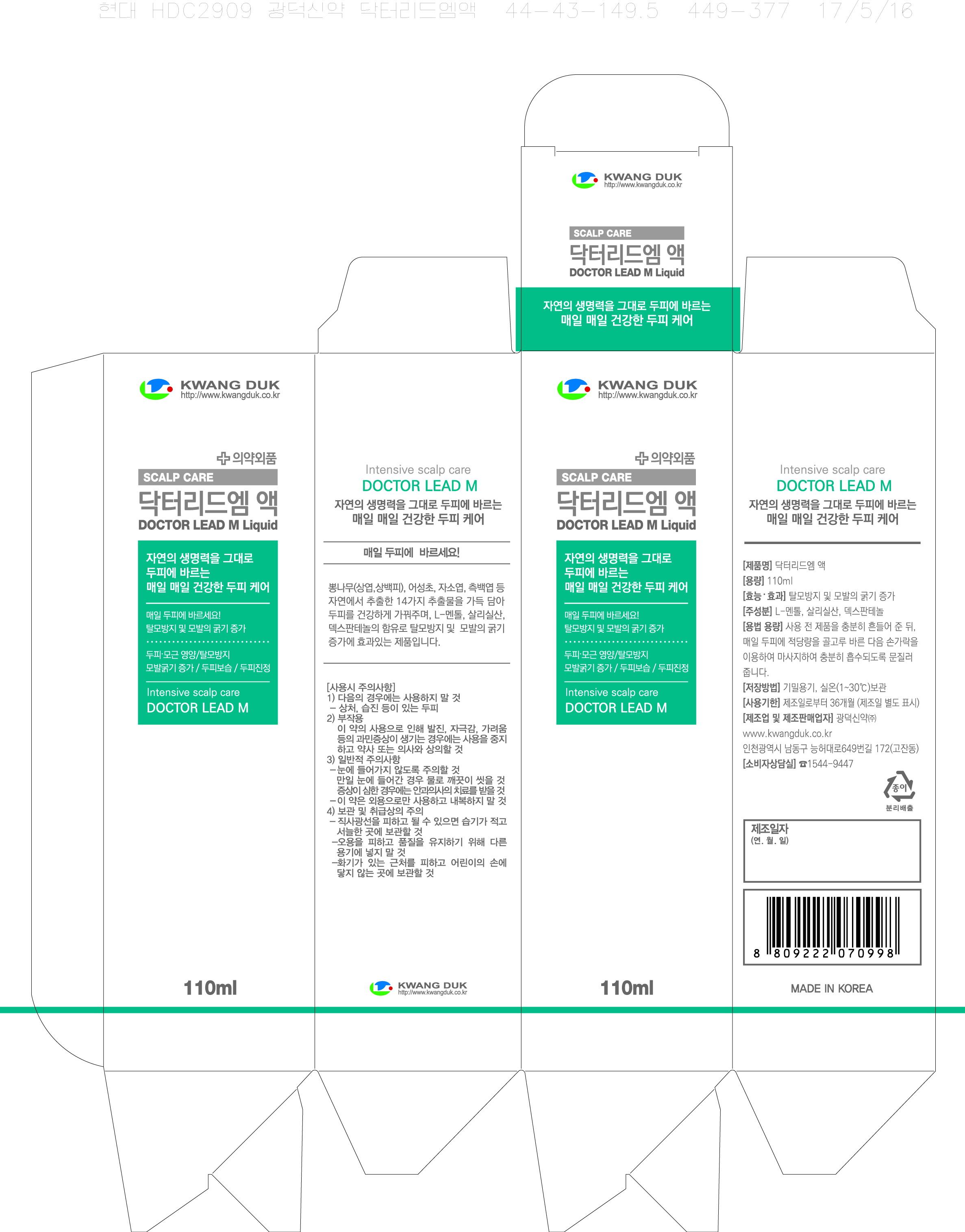
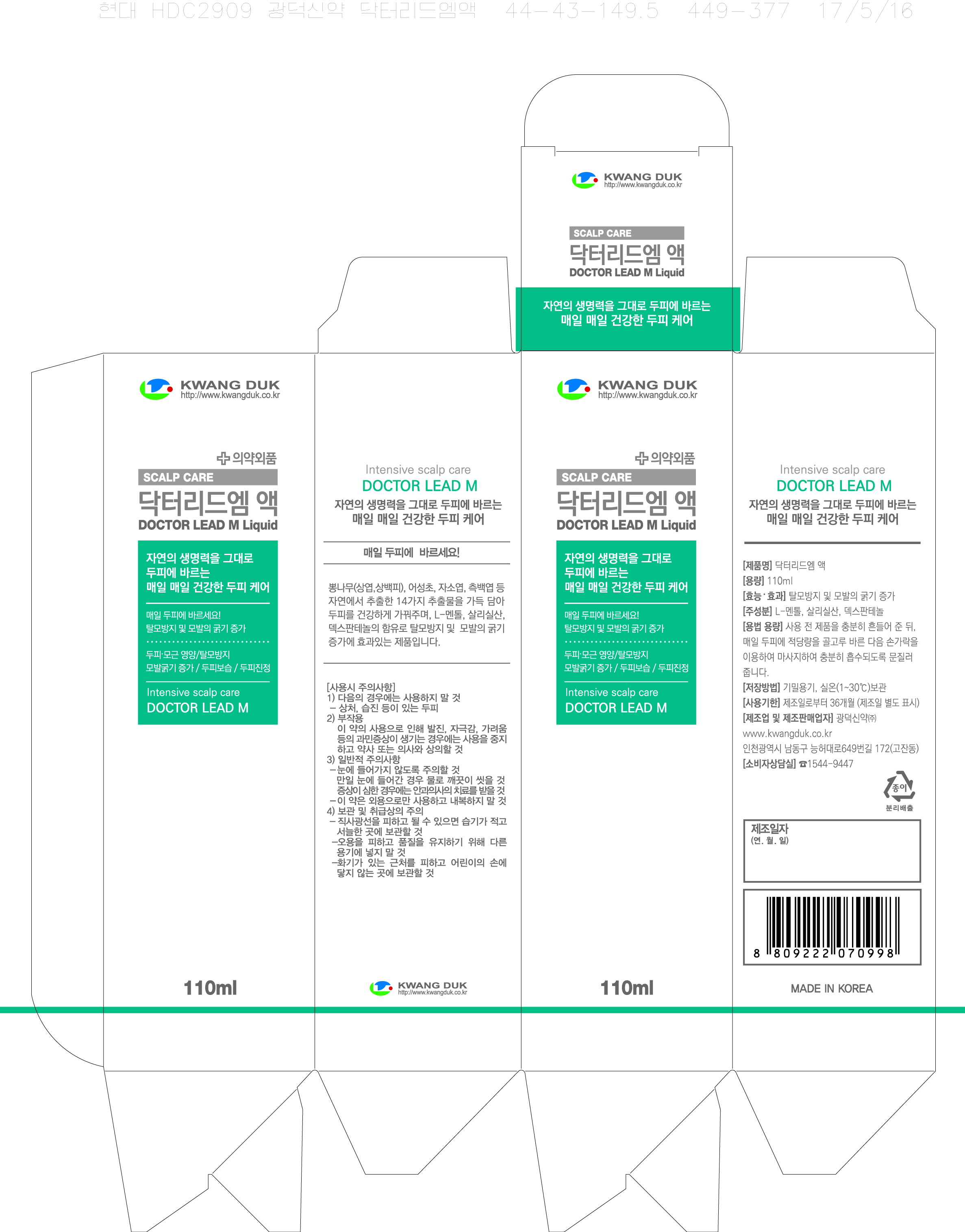
Label: DOCTOR LEAD M- salicylic acid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 71838-0001-1 - Packager: Kwang Duk Sin Yak Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 2, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Cautions :
1. When you use this product and find any of following problems, stop using it. if you keep using it despite the problem, the symptom will get worse, so you will have to go see a dermatologist.
- During use, if you have red spots, swelling, itching, or any other stimuli.
- if the applied region has any problem above for direct light.
2. Do not use the product on wound or dermatitis. 3.Attentive points for storage and treatment :
- Store it in the place where infants or children cannot touch
- do not store it where it has either high or low temperature or there is direct light.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DOCTOR LEAD M
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71838-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 25 g in 100 mL Inactive Ingredients Ingredient Name Strength PANTHENOL (UNII: WV9CM0O67Z) JOJOBA OIL (UNII: 724GKU717M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71838-0001-1 110 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/24/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 10/24/2017 Labeler - Kwang Duk Sin Yak Co., Ltd. (688202646) Registrant - Kwang Duk Sin Yak Co., Ltd. (688202646) Establishment Name Address ID/FEI Business Operations Kwang Duk Sin Yak Co., Ltd. 688202646 manufacture(71838-0001)