Label: ANTICOAGULANT CITRATE DEXTROSE A- anhydrous citric acid, dextrose monohydrate, and trisodium citrate dihydrate solution
- NDC Code(s): 23731-6051-1, 23731-6051-2, 23731-6051-3
- Packager: Citra Labs LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Description
Anticoagulant Citrate Dextrose Solution, Solution A, U.S.P., (ACD-A), is a sterile, non-pyrogenic solution of citric acid, sodium citrate, and dextrose, in water for injection. Each 10 mL of solution contains:
Citric Acid, anhydrous, USP 0.073 g Sodium Citrate, dihydrate, USP 0.220 g Dextrose, monohydrate, USP 0.245 g Water for Injection, USP q.s. pH: 4.5 – 5.5  Single patient use only, on a single occasion.
Single patient use only, on a single occasion.
- Clinical Pharmacology
-
Indications and Usage
Anticoagulant Citrate Dextrose Solution, Solution A, U.S.P. (ACD-A), is intended for use as an anticoagulant in the extracorporeal blood processing with Autologous PRP Systems in production of platelet rich plasma (PRP). Refer to the manufacturer's Operator's Manual of the Autologous PRP System for the Directions for Use.
- Contraindications
- Warnings
-
Precautions
General
- Aseptic technique must be maintained at all times.
- ACD-A solution is a clear and colorless solution. If the product shows any cloudiness or turbidity, the product should be discarded.
- The closure system provides a biological barrier and should be intact – discard product if system is compromised.
Carcinogenesis, mutagenesis, impairment of fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of ACD-A.
- Adverse Reactions
- Overdosage
- Dosage and Administration
- Rx Only
- How Supplied
- SPL UNCLASSIFIED SECTION
-
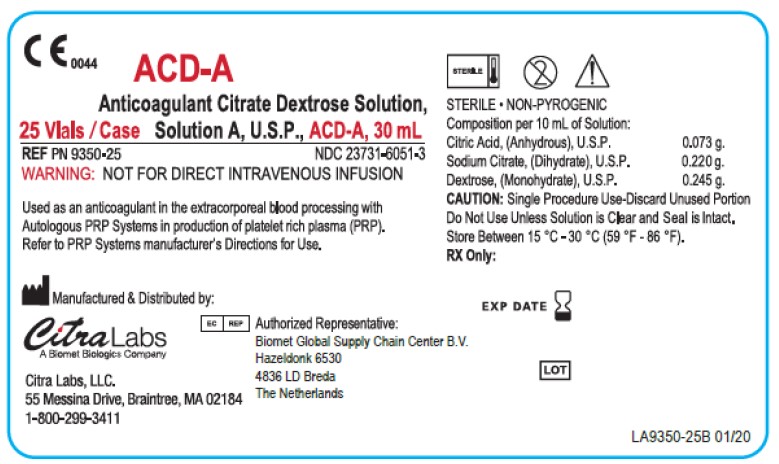
PRINCIPAL DISPLAY PANEL - 10 Vial Case Label
C E 0044
ACD-A
Anticoagulant Citrate Dextrose Solution,
10 Vials / Case Solution A, U.S.P., ACD-A, 30 mLREF PN 9350-10
WARNING: NOT FOR DIRECT INTRAVENOUS INFUSIONUsed as an anticoagulant in the extracorporeal blood processing with
Autologous PRP Systems in production of platelet rich plasma (PRP).
Refer to PRP Systems manufacturer's Directions for Use.Manufactured & Distributed by:
CitraLabs
A Biomet Inc. Company.Citra Labs, LLC.
55 Messina Drive, Braintree, MA 02184
1-800-299-3411EC REP
Authorized Representative:
Biomet Global Supply Chain Center B.V.
Hazeldonk 6530
4836 LD Breda
The Netherlands
STERILE • NON-PYROGENIC
Composition per 10 mL of Solution:
Citric Acid, (Anhydrous), U.S.P. 0.073 g.
Sodium Citrate, (Dihydrate), U.S.P. 0.220 g.
Dextrose, (Monohydrate), U.S.P. 0.245 g.
CAUTION: Single Procedure Use-Discard Unused Portion
Do Not Use Unless Solution is Clear and Seal is Intact.
Store Between 15 °C - 30 °C (59 °F - 86 °F).
RX Only:EXP DATE
LOT
LA9350-10B 01/20

-
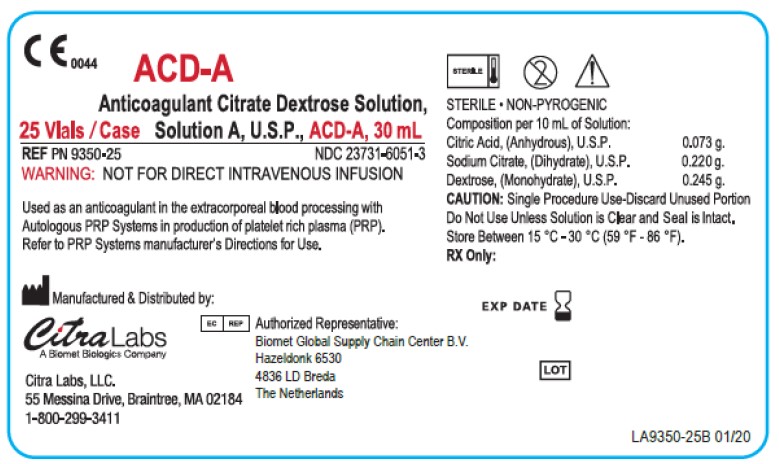
PRINCIPAL DISPLAY PANEL - 25 Vial Case Label
C E 0044
ACD-A
Anticoagulant Citrate Dextrose Solution,
25 Vials / Case Solution A, U.S.P., ACD-A, 30 mLREF PN 9350-25
WARNING: NOT FOR DIRECT INTRAVENOUS INFUSIONUsed as an anticoagulant in the extracorporeal blood processing with
Autologous PRP Systems in production of platelet rich plasma (PRP).
Refer to PRP Systems manufacturer's Directions for Use.Manufactured & Distributed by:
CitraLabs
A Biomet Inc. Company.Citra Labs, LLC.
55 Messina Drive, Braintree, MA 02184
1-800-299-3411EC REP
Authorized Representative:
Biomet Global Supply Chain Center B.V.
Hazeldonk 6530
4836 LD Breda
The Netherlands
STERILE • NON-PYROGENIC
Composition per 10 mL of Solution:
Citric Acid, (Anhydrous), U.S.P. 0.073 g.
Sodium Citrate, (Dihydrate), U.S.P. 0.220 g.
Dextrose, (Monohydrate), U.S.P. 0.245 g.
CAUTION: Single Procedure Use-Discard Unused Portion
Do Not Use Unless Solution is Clear and Seal is Intact.
Store Between 15 °C - 30 °C (59 °F - 86 °F).
RX Only:EXP DATE
LOT
LA9350-25 01/20
-
INGREDIENTS AND APPEARANCE
ANTICOAGULANT CITRATE DEXTROSE A
anhydrous citric acid, dextrose monohydrate, and trisodium citrate dihydrate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:23731-6051 Route of Administration EXTRACORPOREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 0.245 g in 10 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 0.073 g in 10 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 0.22 g in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23731-6051-3 30 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:23731-6051-1 10 in 1 CASE 2 NDC:23731-6051-3 30 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 3 NDC:23731-6051-2 25 in 1 CASE 3 NDC:23731-6051-3 30 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN020037 08/26/2003 Labeler - Citra Labs LLC (962863838) Establishment Name Address ID/FEI Business Operations Citra Labs LLC 962863838 MANUFACTURE(23731-6051)