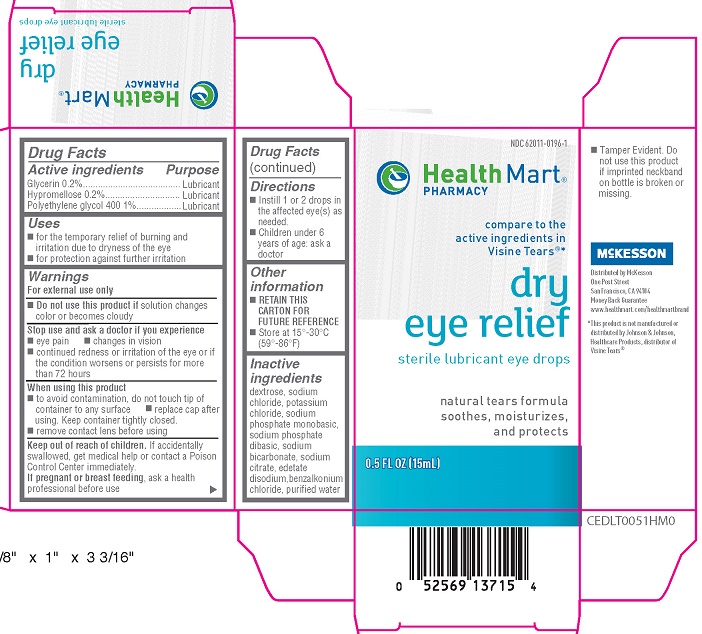
Label: HEALTH MART DRY EYE RELIEF LUBRICANT EYE DROPS- glycerin, hypromellose, polyethylene glycol 400 solution/ drops
- NDC Code(s): 62011-0196-1
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
WARNINGS
Warnings
For external use only
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye or if the condition worsen or persists for more than 72 hours
When using this product
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using. Keep container tightly closed
- remove contact lens before using
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEALTH MART DRY EYE RELIEF LUBRICANT EYE DROPS
glycerin, hypromellose, polyethylene glycol 400 solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62011-0196 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPROMELLOSES (UNII: 3NXW29V3WO) (HYPROMELLOSE, UNSPECIFIED - UNII:3NXW29V3WO) HYPROMELLOSES 0.2 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 0.2 g in 100 mL POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) (POLYETHYLENE GLYCOL 400 - UNII:B697894SGQ) POLYETHYLENE GLYCOL 400 1 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CITRATE (UNII: 1Q73Q2JULR) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) DEXTROSE (UNII: IY9XDZ35W2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62011-0196-1 1 in 1 CARTON 05/01/2013 08/31/2024 1 15 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 05/01/2013 08/31/2024 Labeler - Strategic Sourcing Services LLC (116956644) Registrant - KC Pharmaceuticals, Inc. (174450460) Establishment Name Address ID/FEI Business Operations KC Pharmaceuticals, Inc. 174450460 manufacture(62011-0196) , pack(62011-0196) , label(62011-0196)