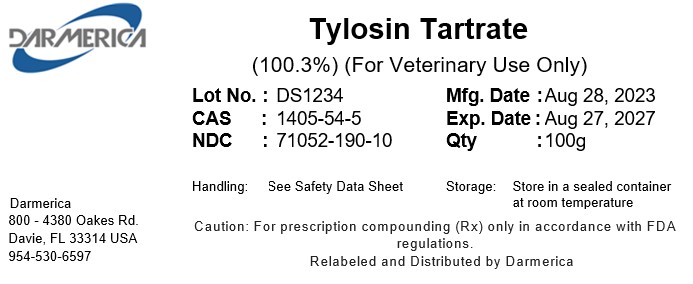
Label: TYLOSIN TARTRATE powder
- NDC Code(s): 71052-190-00, 71052-190-05, 71052-190-10, 71052-190-20
- Packager: DARMERICA, LLC
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: Bulk Ingredient For Animal Drug Compounding
Drug Label Information
Updated January 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Tylosin Tartrate
-
INGREDIENTS AND APPEARANCE
TYLOSIN TARTRATE
tylosin tartrate powderProduct Information Product Type Item Code (Source) NDC:71052-190 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Tylosin Tartrate (UNII: 5P4625C51T) (TYLOSIN - UNII:YEF4JXN031) Tylosin Tartrate 1 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71052-190-20 1 kg in 1 PACKAGE 2 NDC:71052-190-05 5 kg in 1 PACKAGE 3 NDC:71052-190-00 0.5 kg in 1 PACKAGE 4 NDC:71052-190-10 0.10 kg in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date bulk ingredient for animal drug compounding 01/24/2023 Labeler - DARMERICA, LLC (080233052)