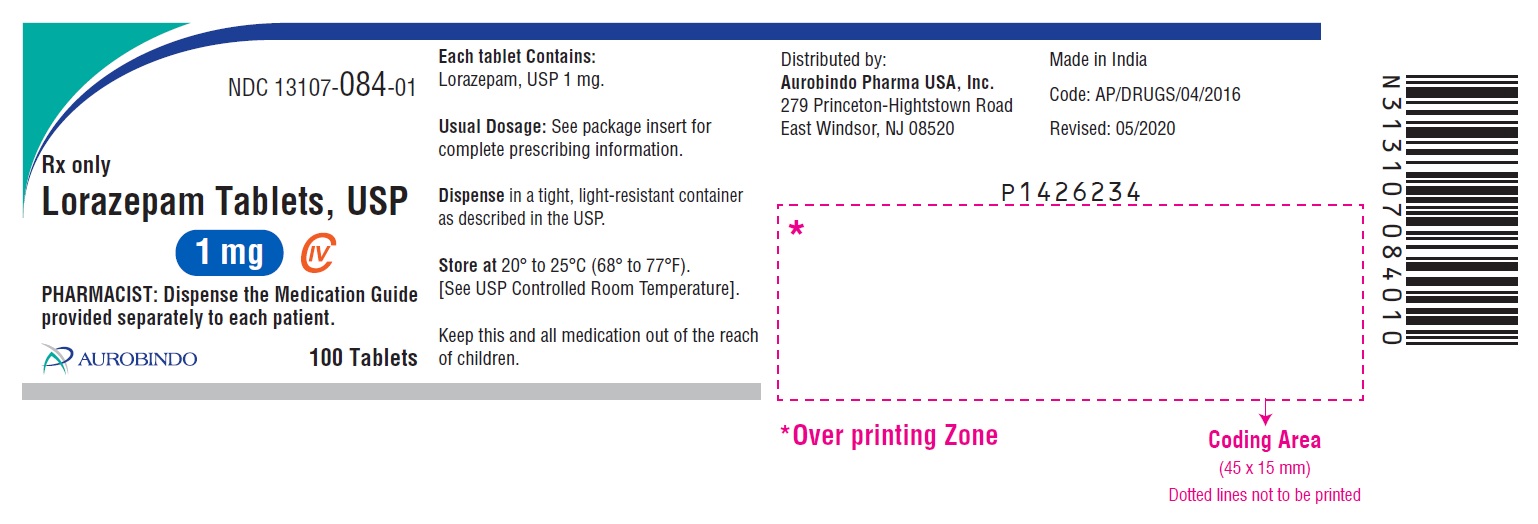
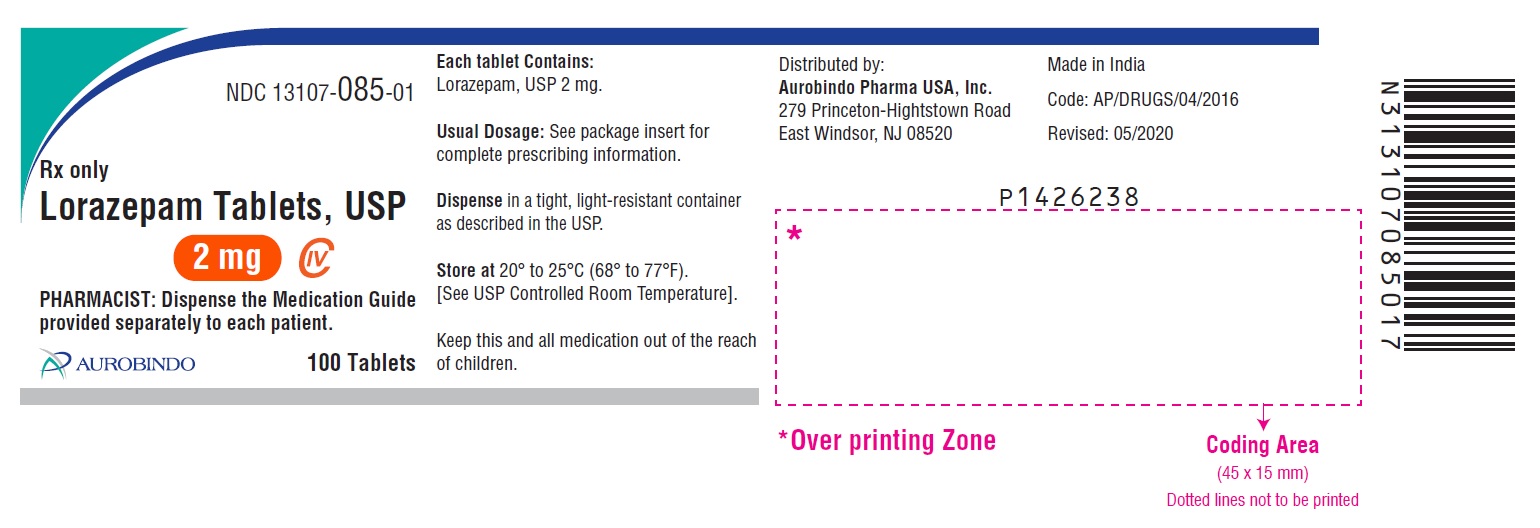
Label: LORAZEPAM tablet
-
NDC Code(s):
13107-083-01,
13107-083-05,
13107-083-10,
13107-083-99, view more13107-084-01, 13107-084-05, 13107-084-10, 13107-084-99, 13107-085-01, 13107-085-05, 13107-085-10, 13107-085-99
- Packager: Aurolife Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated June 7, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS).
- The use of benzodiazepines, including lorazepam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing lorazepam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).
- The continued use of benzodiazepines, including lorazepam, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of lorazepam after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam or reduce the dosage (DOSAGE AND ADMINISTRATION and WARNINGS).
-
DESCRIPTION
Lorazepam USP, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one:
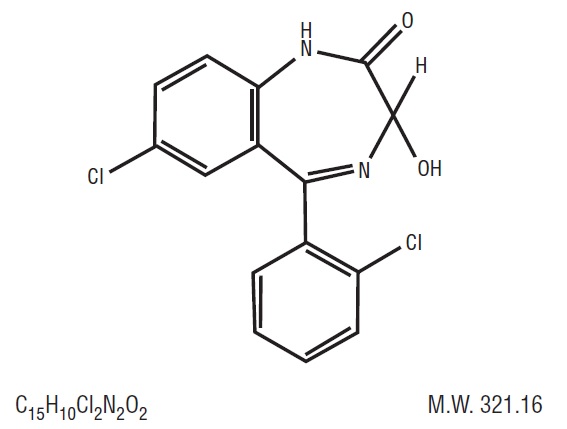
It is a nearly white powder almost insoluble in water. Each lorazepam tablet, USP to be taken orally, contains 0.5 mg, 1 mg, or 2 mg of lorazepam, USP. The inactive ingredients present are anhydrous lactose, magnesium stearate, microcrystalline cellulose, and polacrilin potassium. -
CLINICAL PHARMACOLOGY
Studies in healthy volunteers show that in single high doses lorazepam has a tranquilizing action on the central nervous system with no appreciable effect on the respiratory or cardiovascular systems.
Lorazepam is readily absorbed with an absolute bioavailability of 90%. Peak concentrations in plasma occur approximately 2 hours following administration. The peak plasma level of lorazepam from a 2 mg dose is approximately 20 ng/mL.
The mean half-life of unconjugated lorazepam in human plasma is about 12 hours and for its major metabolite, lorazepam glucuronide, about 18 hours. At clinically relevant concentrations, lorazepam is approximately 85% bound to plasma proteins. Lorazepam is rapidly conjugated at its 3-hydroxy group into lorazepam glucuronide which is then excreted in the urine. Lorazepam glucuronide has no demonstrable central nervous system (CNS) activity in animals.
The plasma levels of lorazepam are proportional to the dose given. There is no evidence of accumulation of lorazepam on administration up to 6 months.
Studies comparing young and elderly subjects have shown that advancing age does not have a significant effect on the pharmacokinetics of lorazepam. However, in one study involving single intravenous doses of 1.5 to 3 mg of lorazepam Injection, mean total body clearance of lorazepam decreased by 20% in 15 elderly subjects of 60 to 84 years of age compared to that in 15 younger subjects of 19 to 38 years of age.
-
INDICATIONS AND USAGE
Lorazepam tablets are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of lorazepam tablets in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
- CONTRAINDICATIONS
-
WARNINGS
Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including lorazepam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe lorazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of lorazepam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking lorazepam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when lorazepam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction
The use of benzodiazepines, including lorazepam, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing lorazepam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of lorazepam, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of lorazepam along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of lorazepam).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including lorazepam, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of lorazepam after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam. Lorazepam is not recommended for use in patients with a primary depressive disorder or psychosis.
Use of benzodiazepines, including lorazepam, both used alone and in combination with other CNS depressants, may lead to potentially fatal respiratory depression (see PRECAUTIONS: Drug Interactions).
As with all patients on CNS-depressant drugs, patients receiving lorazepam should be warned not to operate dangerous machinery or motor vehicles and that their tolerance for alcohol and other CNS depressants will be diminished.
Neonatal Sedation and Withdrawal Syndrome
Use of lorazepam late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see PRECAUTIONS: Pregnancy). Monitor neonates exposed to lorazepam during pregnancy or labor for signs of sedation and monitor neonates exposed to lorazepam during pregnancy for signs of withdrawal; manage these neonates accordingly.
-
PRECAUTIONS
In patients with depression, a possibility for suicide should be borne in mind; benzodiazepines should not be used in such patients without adequate antidepressant therapy.
Lorazepam should be used with caution in patients with compromised respiratory function (e.g., COPD, sleep apnea syndrome).
Elderly or debilitated patients may be more susceptible to the sedative effects of lorazepam. Therefore, these patients should be monitored frequently and have their dosage adjusted carefully according to patient response; the initial dosage should not exceed 2 mg.
Paradoxical reactions have been occasionally reported during benzodiazepine use. Such reactions may be more likely to occur in children and the elderly. Should these occur, use of the drug should be discontinued.
The usual precautions for treating patients with impaired renal or hepatic function should be observed. As with all benzodiazepines, the use of lorazepam may worsen hepatic encephalopathy; therefore, lorazepam should be used with caution in patients with severe hepatic insufficiency and/or encephalopathy. Dosage for patients with severe hepatic insufficiency should be adjusted carefully according to patient response; lower doses may be sufficient in such patients.
In patients where gastrointestinal or cardiovascular disorders coexist with anxiety, it should be noted that lorazepam has not been shown to be of significant benefit in treating the gastrointestinal or cardiovascular component.
Esophageal dilation occurred in rats treated with lorazepam for more than 1 year at 6 mg/kg/day. The no-effect dose was 1.25 mg/kg/day (approximately 6 times the maximum human therapeutic dose of 10 mg/day). The effect was reversible only when the treatment was withdrawn within 2 months of first observation of the phenomenon. The clinical significance of this is unknown. However, use of lorazepam for prolonged periods and in geriatric patients requires caution, and there should be frequent monitoring for symptoms of upper GI disease.
Safety and effectiveness of lorazepam in children of less than 12 years have not been established.
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risks from Concomitant Use with Opioids
Advise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when lorazepam is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see WARNINGS: Risks from Concomitant Use of Opioids and PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction
Inform patients that the use of lorazepam even at recommended doses, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS: Abuse Misuse, and Addiction and DRUG ABUSE AND DEPENDENCE).
Withdrawal Reactions
Inform patients that the continued use of lorazepam may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of lorazepam may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of lorazepam may require a slow taper (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE).
Pregnancy
Advise pregnant females that use of lorazepam late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and PRECAUTIONS: Pregnancy). Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to lorazepam during pregnancy (see PRECAUTIONS: Pregnancy).
Nursing
Instruct patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed. Instruct breastfeeding patients using lorazepam to monitor infants for excessive sedation, poor feeding and poor weight gain, and to seek medical attention if they notice these signs (see PRECAUTIONS: Nursing Mothers).
Essential Laboratory Tests
Some patients on lorazepam have developed leukopenia, and some have had elevations of LDH. As with other benzodiazepines, periodic blood counts and liver function tests are recommended for patients on long-term therapy.
Drug Interactions
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
The benzodiazepines, including lorazepam, produce increased CNS-depressant effects when administered with other CNS depressants such as alcohol, barbiturates, antipsychotics, sedative/hypnotics, anxiolytics, antidepressants, narcotic analgesics, sedative antihistamines, anticonvulsants, and anesthetics.
Concomitant use of clozapine and lorazepam may produce marked sedation, excessive salivation, hypotension, ataxia, delirium, and respiratory arrest.
Concurrent administration of lorazepam with valproate results in increased plasma concentrations and reduced clearance of lorazepam. Lorazepam dosage should be reduced to approximately 50% when coadministered with valproate.
Concurrent administration of lorazepam with probenecid may result in a more rapid onset or prolonged effect of lorazepam due to increased half-life and decreased total clearance. Lorazepam dosage needs to be reduced by approximately 50% when coadministered with probenecid.
The effects of probenecid and valproate on lorazepam may be due to inhibition of glucuronidation.
Administration of theophylline or aminophylline may reduce the sedative effects of benzodiazepines, including lorazepam.
Carcinogenesis and Mutagenesis
No evidence of carcinogenic potential emerged in rats during an 18-month study with lorazepam. No studies regarding mutagenesis have been performed.
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including lorazepam, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and Clinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to lorazepam during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to lorazepam during pregnancy for signs of withdrawal. Manage these neonates accordingly (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome).
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Animal Data
Reproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg and higher, there was evidence of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
Nursing Mothers
Risk Summary
Lorazepam is present in breast milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. The effects of lorazepam on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for lorazepam and any potential adverse effects on the breastfed infant from lorazepam or from the underlying maternal condition.
Clinical Considerations
Infants exposed to lorazepam through breast milk should be monitored for sedation, poor feeding and poor weight gain.
Geriatric Use
Clinical studies of lorazepam generally were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects; however, the incidence of sedation and unsteadiness was observed to increase with age (see ADVERSE REACTIONS).
Age does not appear to have a significant effect on lorazepam kinetics (see CLINICAL PHARMACOLOGY).
Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity (e.g., sedation) of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious, and lower doses may be sufficient in these patients (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Most adverse reactions to benzodiazepines, including CNS effects and respiratory depression, are dose dependent, with more severe effects occurring with high doses.
In a sample of about 3500 patients treated for anxiety, the most frequent adverse reaction to lorazepam was sedation (15.9%), followed by dizziness (6.9%), weakness (4.2%), and unsteadiness (3.4%). The incidence of sedation and unsteadiness increased with age.
Other adverse reactions to benzodiazepines, including lorazepam are fatigue, drowsiness, amnesia, memory impairment, confusion, disorientation, depression, unmasking of depression, disinhibition, euphoria, suicidal ideation/attempt, ataxia, asthenia, extrapyramidal symptoms, convulsions/seizures, tremor, vertigo, eye function/visual disturbance (including diplopia and blurred vision), dysarthria/slurred speech, change in libido, impotence, decreased orgasm; headache, coma; respiratory depression, apnea, worsening of sleep apnea, worsening of obstructive pulmonary disease; gastrointestinal symptoms including nausea, change in appetite, constipation, jaundice, increase in bilirubin, increase in liver transaminases, increase in alkaline phosphatase; hypersensitivity reactions, anaphylactoid reactions; dermatological symptoms, allergic skin reactions, alopecia; syndrome of inappropriate antidiuretic hormone (SIADH), hyponatremia; thrombocytopenia, agranulocytosis, pancytopenia; hypothermia; and autonomic manifestations.
Paradoxical reactions, including anxiety, excitation, agitation, hostility, aggression, rage, sleep disturbances/insomnia, sexual arousal, and hallucinations may occur. Small decreases in blood pressure and hypotension may occur but are usually not clinically significant, probably being related to the relief of anxiety produced by lorazepam.
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -
DRUG ABUSE AND DEPENDENCE
Abuse
Lorazepam is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a healthcare provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse of benzodiazepines may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS: Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Dependence
Physical Dependence
Lorazepam may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (see WARNINGS: Dependence and Withdrawal Reactions).
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam or reduce the dosage (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of lorazepam and WARNINGS).
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to lorazepam may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of lorazepam may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
-
OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal (see WARNINGS: Abuse, Misuse , and Addiction). Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
-
DOSAGE AND ADMINISTRATION
Lorazepam tablets are administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available.
The usual range is 2 to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 to 10 mg/day.
For anxiety, most patients require an initial dose of 2 to 3 mg/day given two times a day or three times a day.
For insomnia due to anxiety or transient situational stress, a single daily dose of 2 to 4 mg may be given, usually at bedtime.
For elderly or debilitated patients, an initial dosage of 1 to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated.
The dosage of lorazepam tablets should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
Discontinuation or Dosage Reduction of Lorazepam Tablets
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue lorazepam tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE: Dependence).
-
HOW SUPPLIED
Lorazepam Tablets, USP are available in the following dosage strengths:
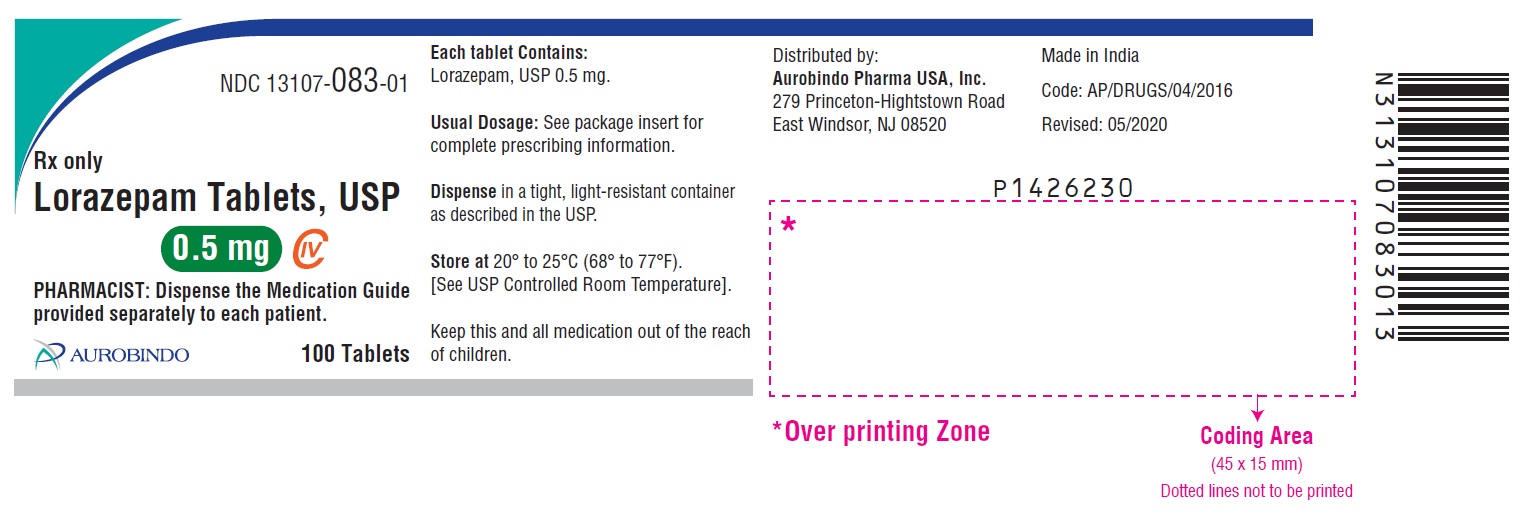
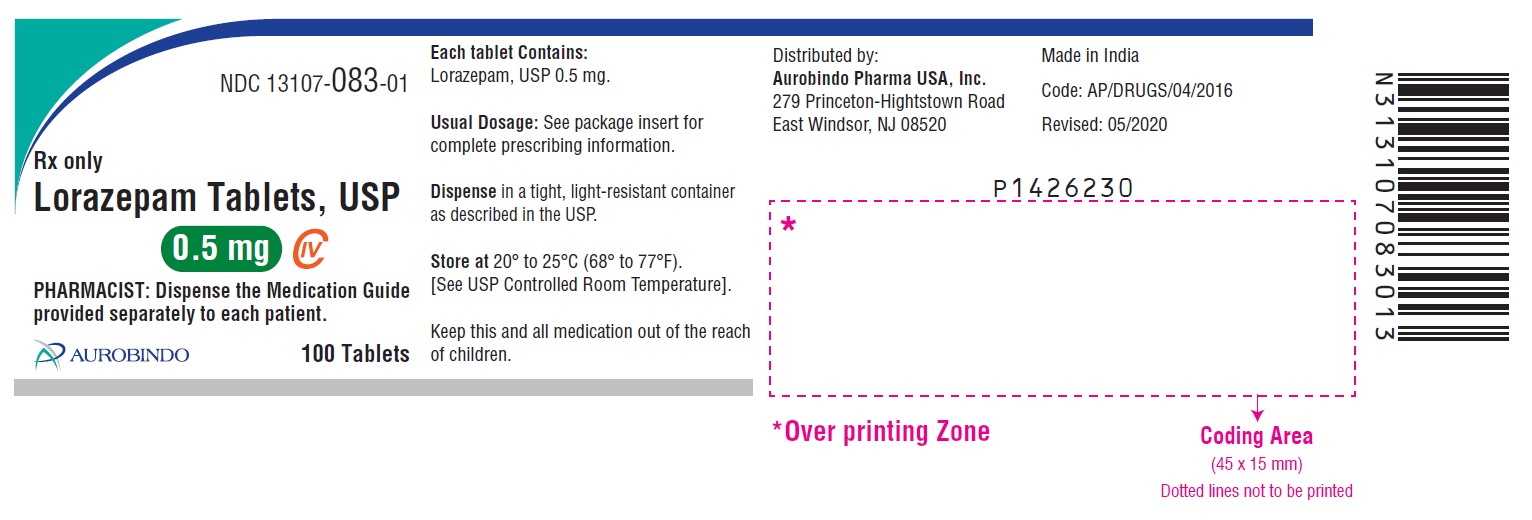
0.5 mg, white to off-white, round, flat-faced beveled edge tablets debossed with ‘U32’ on one side and plain on the other side.
Bottles of 10 tablets NDC 13107-083-11
Bottles of 100 tablets NDC 13107-083-01
Bottles of 500 tablets NDC 13107-083-05
Bottles of 1000 tablets NDC 13107-083-99
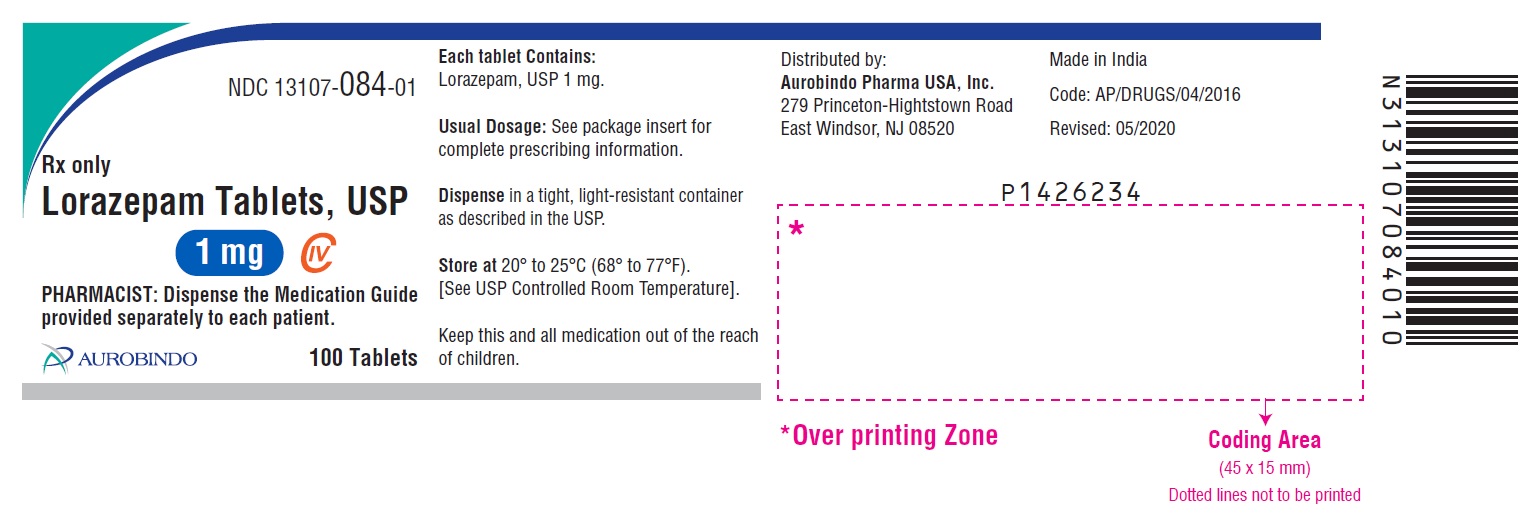
1 mg, white to off-white, round, flat-faced beveled edge tablets debossed with ‘U33’ on one side and bisect on the other side.
Bottles of 10 tablets NDC 13107-084-11
Bottles of 100 tablets NDC 13107-084-01
Bottles of 500 tablets NDC 13107-084-05
Bottles of 1000 tablets NDC 13107-084-99
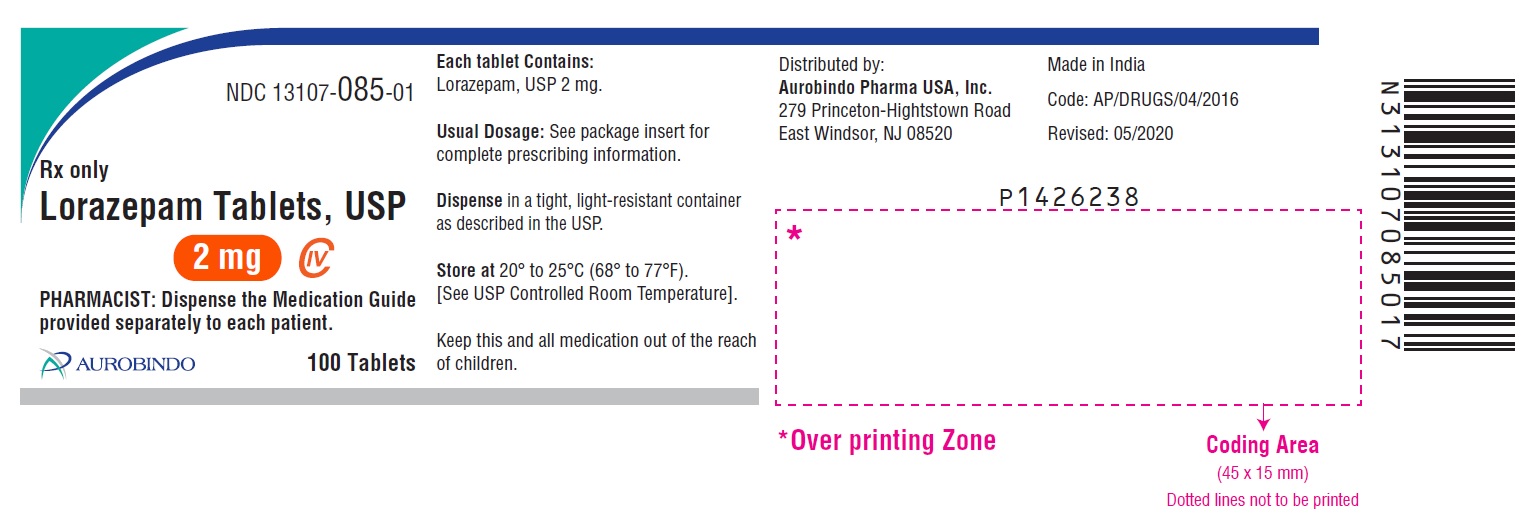
2 mg, white to off-white, round, flat-faced beveled edge tablets debossed with a bisect separating “U” and “34” on one side and “2” on other side.
Bottles of 10 tablets NDC 13107-085-11
Bottles of 100 tablets NDC 13107-085-01
Bottles of 500 tablets NDC 13107-085-05
Bottles of 1000 tablets NDC 13107-085-99
Keep bottles tightly closed.
Keep out of reach of children.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as described in the USP.
Dispense with Medication Guide available at: www.aurobindousa.com/medication-guides
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Revised: 06/2023
Dispense with Medication Guide available at: www.aurobindousa.com/medication-guides -
MEDICATION GUIDE
MEDICATION GUIDE
Lorazepam Tablets, USP CIV
(lor-A-ze-pam)
What is the most important information I should know about lorazepam tablets?
-
Lorazepam tablets are a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
- shallow or slowed breathing
- breathing stops (which may lead to the heart stopping)
- excessive sleepiness (sedation)
Do not drive or operate heavy machinery until you know how taking lorazepam tablets with opioids affects you.
-
Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines including lorazepam tablets which can lead to overdose and serious side effects including coma and death.
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including lorazepam tablets. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- You can develop an addiction even if you take lorazepam tablets exactly as prescribed by your healthcare provider.
- Take lorazepam tablets exactly as your healthcare provider prescribed.
- Do not share your lorazepam tablets with other people.
- Keep lorazepam tablets in a safe place and away from children.
-
Physical dependence and withdrawal reactions. Lorazepam tablets can cause physical dependence and withdrawal reactions.
- Do not suddenly stop taking lorazepam tablets. Stopping lorazepam tablets suddenly can cause serious and life-threatening side effects, including, unusual movements, responses or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
- Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not take more lorazepam tablets than prescribed or take lorazepam tablets for longer than prescribed.
What are lorazepam tablets?
- Lorazepam tablets are a prescription medicine used:
- to treat anxiety disorders
- for the short-term relief of the symptoms of anxiety or anxiety that can happen with symptoms of depression
- Lorazepam tablets are a federal controlled substance (CIV) because it contains lorazepam that can be abused or lead to dependence. Keep lorazepam tablets in a safe place to prevent misuse and abuse. Selling or giving away lorazepam tablets may harm others, and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription medicines or street drugs.
- It is not known if lorazepam tablets are safe and effective for use in children less than 12 years of age.
- It is not known if lorazepam tablets are safe and effective for use for longer than 4 months.
Do not take lorazepam tablets if you:
- are allergic to lorazepam, other benzodiazepines, or any of the ingredients in lorazepam tablets. See the end of this Medication Guide for a complete list of ingredients in lorazepam tablets.
Before you take lorazepam tablets, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have a history of drug or alcohol abuse or addiction
- have lung disease or breathing problems (such as COPD, sleep apnea syndrome)
- have liver or kidney problems
- have or have had seizures
- are pregnant or plan to become pregnant.
- Taking lorazepam tablets late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with lorazepam tablets.
- There is a pregnancy registry for women who take lorazepam tablets during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. If you become pregnant during treatment with lorazepam tablets, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychiatric Medications. You can register by calling 1-866-961-2388 or visiting https://womensmentalhealth.org/pregnancyregistry/.
- are breastfeeding or plan to breastfeed. Lorazepam passes into your breast milk.
- Breastfeeding during treatment with lorazepam tablets may cause your baby to have sleepiness, feeding problems, and decreased weight gain.
- Talk to your healthcare provider about the best way to feed your baby if you take lorazepam tablets.
Taking lorazepam tablets with certain other medicines can cause side effects or affect how well lorazepam tablets or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I take lorazepam tablets?
- Take lorazepam tablets exactly as your healthcare provider tells you to take it. Your healthcare provider will tell you how much lorazepam tablets to take and when to take it.
- If you take too much lorazepam tablets, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of lorazepam tablets?
Lorazepam tablets may cause serious side effects, including:
- See “What is the most important information I should know about lorazepam tablets?”
-
Lorazepam tablets can make you sleepy or dizzy and can slow your thinking and motor skills.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how lorazepam tablets affects you.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking lorazepam tablets without first talking to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, lorazepam tablets may make your sleepiness or dizziness much worse.
- Depression. Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam tablets.
The most common side effects of lorazepam tablets include:
• sedation • dizziness
• weakness • unsteadiness
These are not all the possible side effects of lorazepam tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lorazepam tablets?
- Store lorazepam tablets in a tightly closed container at room temperature between 20° to 25°C (68° to 77°F).
- Keep lorazepam tablets and all medicines out of the reach of children.
General information about the safe and effective use of lorazepam tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use lorazepam tablets for a condition for which it was not prescribed. Do not give lorazepam tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about lorazepam tablets that is written for health professionals.
What are the ingredients in lorazepam tablets?
Active ingredient: lorazepam
Inactive ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, and polacrilin potassium.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 06/2023
-
Lorazepam tablets are a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.5 mg 100 Tablets Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg 100 Tablets Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 2 mg 100 Tablets Bottle)
-
INGREDIENTS AND APPEARANCE
LORAZEPAM
lorazepam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:13107-083 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORAZEPAM (UNII: O26FZP769L) (LORAZEPAM - UNII:O26FZP769L) LORAZEPAM 0.5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) Product Characteristics Color WHITE (White to off white) Score no score Shape ROUND (Flat-faced beveled edge) Size 5mm Flavor Imprint Code U32 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13107-083-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 2 NDC:13107-083-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 3 NDC:13107-083-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 4 NDC:13107-083-99 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203572 03/24/2017 LORAZEPAM
lorazepam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:13107-084 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORAZEPAM (UNII: O26FZP769L) (LORAZEPAM - UNII:O26FZP769L) LORAZEPAM 1 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) Product Characteristics Color WHITE (White to off white) Score 2 pieces Shape ROUND (Flat-faced beveled edge) Size 7mm Flavor Imprint Code U33 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13107-084-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 2 NDC:13107-084-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 3 NDC:13107-084-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 4 NDC:13107-084-99 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203572 03/24/2017 LORAZEPAM
lorazepam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:13107-085 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORAZEPAM (UNII: O26FZP769L) (LORAZEPAM - UNII:O26FZP769L) LORAZEPAM 2 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) Product Characteristics Color WHITE (White to off white) Score 2 pieces Shape ROUND (Flat-faced beveled edge) Size 7mm Flavor Imprint Code U34 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13107-085-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 2 NDC:13107-085-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 3 NDC:13107-085-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 4 NDC:13107-085-99 1000 in 1 BOTTLE; Type 0: Not a Combination Product 03/24/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203572 03/24/2017 Labeler - Aurolife Pharma LLC (829084461) Establishment Name Address ID/FEI Business Operations Aurolife Pharma LLC 829084461 ANALYSIS(13107-083) , MANUFACTURE(13107-083, 13107-084, 13107-085) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650918514 ANALYSIS(13107-083, 13107-084, 13107-085) , MANUFACTURE(13107-083, 13107-084, 13107-085)