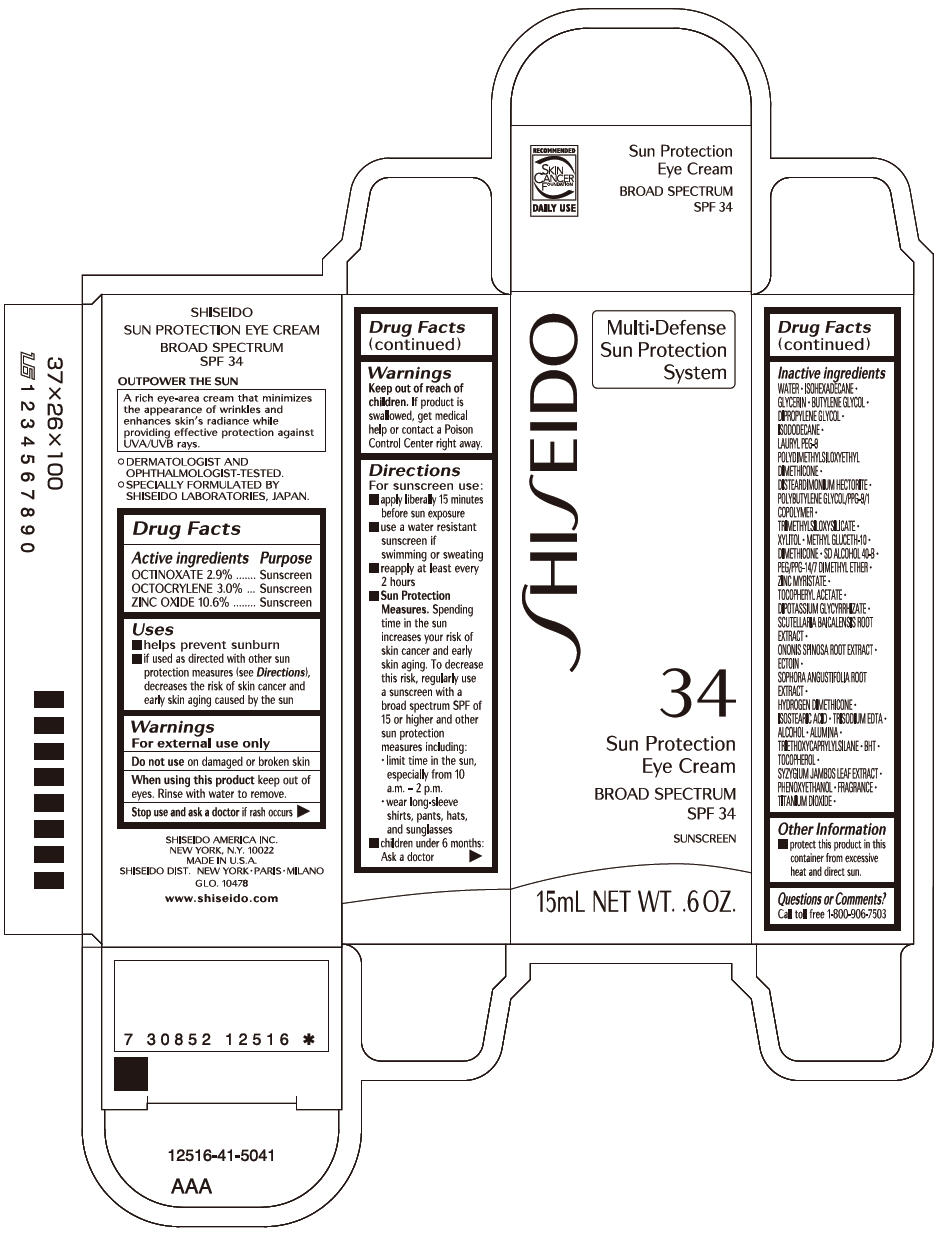
Label: SHISEIDO SUN PROTECTION EYE- octinoxate, octocrylene, and zinc oxide cream
- NDC Code(s): 52686-335-60
- Packager: SHISEIDO AMERICA INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
WATER • ISOHEXADECANE • GLYCERIN • BUTYLENE GLYCOL • DIPROPYLENE GLYCOL • ISODODECANE • LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE • DISTEARDIMONIUM HECTORITE • POLYBUTYLENE GLYCOL/PPG-9/1 COPOLYMER • TRIMETHYLSILOXYSILICATE • XYLITOL • METHYL GLUCETH-10 • DIMETHICONE • SD ALCOHOL 40-B • PEG/PPG-14/7 DIMETHYL ETHER • ZINC MYRISTATE • TOCOPHERYL ACETATE • DIPOTASSIUM GLYCYRRHIZATE • SCUTELLARIA BAICALENSIS ROOT EXTRACT • ONONIS SPINOSA ROOT EXTRACT • ECTOIN • SOPHORA ANGUSTIFOLIA ROOT EXTRACT • HYDROGEN DIMETHICONE • ISOSTEARIC ACID • TRISODIUM EDTA • ALCOHOL • ALUMINA • TRIETHOXYCAPRYLYLSILANE • BHT • TOCOPHEROL • SYZYGIUM JAMBOS LEAF EXTRACT • PHENOXYETHANOL • FRAGRANCE • TITANIUM DIOXIDE •
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 15mL Tube Carton
-
INGREDIENTS AND APPEARANCE
SHISEIDO SUN PROTECTION EYE
octinoxate, octocrylene, and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52686-335 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 479 mg in 16.5 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 495 mg in 16.5 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1749 mg in 16.5 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOHEXADECANE (UNII: 918X1OUF1E) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIPROPYLENE GLYCOL (UNII: E107L85C40) ISODODECANE (UNII: A8289P68Y2) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) XYLITOL (UNII: VCQ006KQ1E) METHYL GLUCETH-10 (UNII: N0MWT4C7WH) DIMETHICONE (UNII: 92RU3N3Y1O) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) ZINC MYRISTATE (UNII: K09A9E2GGO) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) ONONIS SPINOSA ROOT (UNII: FD2FMC53M1) ECTOINE (UNII: 7GXZ3858RY) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) ISOSTEARIC ACID (UNII: X33R8U0062) EDETATE TRISODIUM (UNII: 420IP921MB) ALCOHOL (UNII: 3K9958V90M) ALUMINUM OXIDE (UNII: LMI26O6933) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) SYZYGIUM JAMBOS LEAF (UNII: 407Z4W5LFF) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52686-335-60 1 in 1 CARTON 12/01/2012 11/01/2015 1 16.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 12/01/2012 Labeler - SHISEIDO AMERICA INC. (782677132) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 manufacture(52686-335) , analysis(52686-335)