Label: METHIMAZOLE tablet
-
NDC Code(s):
62135-205-01,
62135-205-05,
62135-205-18,
62135-205-30, view more62135-205-90, 62135-206-01, 62135-206-18, 62135-206-30, 62135-206-90
- Packager: Chartwell RX, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Methimazole, USP (1-methylimidazole-2-thiol) is a white, crystalline substance that is freely soluble in water. It differs chemically from the drugs of the thiouracil series primarily because it has a 5- instead of a 6-membered ring.
Each tablet contains 5 mg or 10 mg (43.8 mcmol or 87.6 mcmol) methimazole USP, an orally administered antithyroid drug.
Each tablet also contains anhydrous lactose, colloidal silicon dioxide, lactose monohydrate, magnesium stearate, pregelatinized starch (corn) and talc.
The molecular weight is 114.16, and the molecular formula is C 4H 6N 2S. The structural formula is as follows:
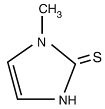
-
CLINICAL PHARMACOLOGY
Methimazole inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. The drug does not inactivate existing thyroxine and tri-iodothyronine that are stored in the thyroid or circulating in the blood nor does it interfere with the effectiveness of thyroid hormones given by mouth or by injection.
Methimazole is readily absorbed in the gastrointestinal tract, metabolized in the liver, and excreted in the urine.
-
INDICATIONS AND USAGE
Methimazole tablets, USP are indicated:
- In patients with Graves’ disease with hyperthyroidism or toxic multinodular goiter for whom surgery or radioactive iodine therapy is not an appropriate treatment option.
- To ameliorate symptoms of hyperthyroidism in preparation for thyroidectomy or radioactive iodine therapy.
- CONTRAINDICATIONS
-
WARNINGS
First Trimester Use of Methimazole and Congenital Malformations
Methimazole crosses the placental membranes and can cause fetal harm, when administered in the first trimester of pregnancy. Rare instances of congenital defects, including aplasia cutis, craniofacial malformations (facial dysmorphism; choanal atresia) gastrointestinal malformations (esophageal atresia with or without tracheoesophageal fistula) omphalocele and abnormalities of the omphalomesenteric duct have occurred in infants born to mothers who received methimazole in the first trimester of pregnancy. If methimazole is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be warned of the potential hazard to the fetus.
Because of the risk for congenital malformations associated with use of methimazole in the first trimester of pregnancy it may be appropriate to use other agents in pregnant women requiring treatment for hyperthyroidism. If methimazole is used, the lowest possible dose to control the maternal disease should be given.
Agranulocytosis
Agranulocytosis is potentially a life-threatening adverse reaction of methimazole therapy. Patients should be instructed to immediately report to their physicians any symptoms suggestive of agranulocytosis, such as fever or sore throat. Leukopenia, thrombocytopenia, and aplastic anemia (pancytopenia) may also occur. The drug should be discontinued in the presence of agranulocytosis or aplastic anemia (pancytopenia), and the patient’s bone marrow indices should be monitored.
Liver Toxicity
Although there have been reports of hepatotoxicity (including acute liver failure) associated with methimazole, the risk of hepatotoxicity appears to be less with methimazole than with propylthiouracil, especially in the pediatric population. Symptoms suggestive of hepatic dysfunction (anorexia, pruritis, right upper quadrant pain, etc.) should prompt evaluation of liver function (bilirubin, alkaline phosphatase) and hepatocellular integrity (ALT, AST). Drug treatment should be discontinued promptly in the event of clinically significant evidence of liver abnormality including hepatic transaminase values exceeding 3 times the upper limit of normal.
Hypothyroidism
Methimazole can cause hypothyroidism necessitating routine monitoring of TSH and free T4 levels with adjustments in dosing to maintain a euthyroid state. Because the drug readily crosses placental membranes, methimazole can cause fetal goiter and cretinism when administered to a pregnant woman. For this reason, it is important that a sufficient, but not excessive, dose be given during pregnancy (see PRECAUTIONS, Pregnancy).
Vasculitis
Cases of vasculitis resulting in severe complications have been reported in patients receiving Methimazole therapy. These cases of vasculitis include: leukocytoclastic cutaneous vasculitis, acute kidney injury and glomerulonephritis, alveolar/pulmonary hemorrhage, CNS vasculitis, and neuropathy. Most cases were associated with anti-neutrophilic cytoplasmic antibodies (ANCA)-positive vasculitis. In some cases, vasculitis resolved/improved with drug discontinuation; however, more severe cases required treatment with additional measures including corticosteroids, immunosuppressant therapy, and plasmapheresis. If vasculitis is suspected, discontinue therapy and initiate appropriate intervention.
-
PRECAUTIONS
General
Patients who receive methimazole should be under close surveillance and should be cautioned to report immediately any evidence of illness, particularly sore throat, skin eruptions, fever, headache, or general malaise. In such cases, white-blood-cell and differential counts should be obtained to determine whether agranulocytosis has developed. Particular care should be exercised with patients who are receiving additional drugs known to cause agranulocytosis.
Information for Patients
Inform patients that cases of vasculitis resulting in severe complications have occurred with Methimazole. Inform patients to promptly report symptoms that may be associated with vasculitis including new rash, hematuria or decreased urine output, dyspnea or hemoptysis (see WARNINGS and ADVERSE REACTIONS). Patients should be advised that if they become pregnant or intend to become pregnant while taking an antithyroid drug, they should contact their physician immediately about their therapy.
Laboratory Tests
Because methimazole may cause hypoprothrombinemia and bleeding, prothrombin time should be monitored during therapy with the drug, especially before surgical procedures.
Thyroid function tests should be monitored periodically during therapy. Once clinical evidence of hyperthyroidism has resolved, the finding of a rising serum TSH indicates that a lower maintenance dose of methimazole should be employed.
Drug Interactions
Anticoagulants (oral)
Due to potential inhibition of vitamin K activity by methimazole, the activity of oral anticoagulants (e.g., warfarin) may be increased; additional monitoring of PT/INR should be considered, especially before surgical procedures.
ß−Adrenergic Blocking Agents
Hyperthyroidism may cause an increased clearance of beta blockers with a high extraction ratio. A dose reduction of beta-adrenergic blockers may be needed when a hyperthyroid patient becomes euthyroid.
Digitalis Glycosides
Serum digitalis levels may be increased when hyperthyroid patients on a stable digitalis glycoside regimen become euthyroid; a reduced dosage of digitalis glycosides may be needed.
Theophylline
Theophylline clearance may decrease when hyperthyroid patients on a stable theophylline regimen become euthyroid; a reduced dose of theophylline may be needed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2 year study, rats were given methimazole at doses of 0.5 mg/kg/day, 3 mg/kg/day and 18 mg/kg/day. These doses were 0.3, 2, and 12 times the 15 mg/day maximum human maintenance dose (when calculated on the basis of surface area). Thyroid hyperplasia, adenoma, and carcinoma developed in rats at the two higher doses. The clinical significance of these findings is unclear.
Pregnancy Category D
See WARNINGS.
If methimazole is used during the first trimester of pregnancy or if the patient becomes pregnant while taking this drug, the patient should be warned of the potential hazard to the fetus.
In pregnant women with untreated or inadequately treated Graves’ disease, there is an increased risk of adverse events of maternal heart failure, spontaneous abortion, preterm birth, stillbirth and fetal or neonatal hyperthyroidism.
Because methimazole crosses placental membranes and can induce goiter and cretinism in the developing fetus, hyperthyroidism should be closely monitored in pregnant women and treatment adjusted such that a sufficient, but not excessive, dose be given during pregnancy. In many pregnant women, the thyroid dysfunction diminishes as the pregnancy proceeds; consequently, a reduction of dosage may be possible. In some instances, anti-thyroid therapy can be discontinued several weeks or months before delivery.
Due to the rare occurrence of congenital malformations associated with methimazole use, it may be appropriate to use an alternative anti-thyroid medication in pregnant women requiring treatment for hyperthyroidism particularly in the first trimester of pregnancy during organogenesis.
Given the potential maternal adverse effects of propylthiouracil (e.g., hepatotoxicity), it may be preferable to switch from propylthiouracil to methimazole for the second and third trimesters.
Nursing Mothers
Methimazole is present in breast milk. However, several studies found no effect on clinical status in nursing infants of mothers taking methimazole. A long-term study of 139 thyrotoxic lactating mothers and their infants failed to demonstrate toxicity in infants who are nursed by mothers receiving treatment with methimazole. Monitor thyroid function at frequent (weekly or biweekly) intervals.
Pediatric Use
Because of postmarketing reports of severe liver injury in pediatric patients treated with propylthiouracil, methimazole is the preferred choice when an antithyroid drug is required for a pediatric patient (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Major adverse reactions (which occur with much less frequency than the minor adverse reactions) include inhibition of myelopoieses (agranulocytosis, granulocytopenia, thrombocytopenia, and aplastic anemia), drug fever, a lupus-like syndrome, insulin autoimmune syndrome (which can result in hypoglycemic coma), hepatitis (jaundice may persist for several weeks after discontinuation of the drug), periarteritis, and hypoprothrombinemia. Nephritis occurs very rarely.
Minor adverse reactions include skin rash, urticaria, nausea, vomiting, epigastric distress, arthralgia, paresthesia, loss of taste, abnormal loss of hair, myalgia, headache, pruritus, drowsiness, neuritis, edema, vertigo, skin pigmentation, jaundice, sialadenopathy, and lymphadenopathy.
There are reports of a vasculitis, often associated with the presence of anti-neutrophilic cytoplasmic antibodies (ANCA), resulting in severe complications (see WARNINGS).
-
OVERDOSAGE
Signs and Symptoms
Symptoms may include nausea, vomiting, epigastric distress, headache, fever, joint pain, pruritus, and edema. Aplastic anemia (pancytopenia) or agranulocytosis may be manifested in hours to days. Less frequent events are hepatitis, nephrotic syndrome, exfoliative dermatitis, neuropathies, and CNS stimulation or depression.
No information is available on the median lethal dose of the drug or the concentration of methimazole in biologic fluids associated with toxicity and/or death.
Treatment
To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in the patient.
In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s medical status.
-
DOSAGE AND ADMINISTRATION
Methimazole tablets, USP are administered orally. The total daily dosage is usually given in 3 divided doses at approximately 8-hour intervals.
Adult
The initial daily dosage is 15 mg for mild hyperthyroidism, 30 mg to 40 mg for moderately severe hyperthyroidism and 60 mg for severe hyperthyroidism, divided into 3 doses at 8-hour intervals. The maintenance dosage is 5 mg to 15 mg daily.
Pediatric
Initially, the daily dosage is 0.4 mg/kg of body weight divided into 3 doses and given at 8-hour intervals. The maintenance dosage is approximately 1/2 of the initial dose.
-
HOW SUPPLIED
Methimazole Tablets, USP, for oral administration, are available as
5 mg
White, round scored tablet, de-bossed with "CE" over "36" on one side and functional score on the other side and supplied as:
NDC 62135-205-30 bottles of 30
NDC 62135-205-90 bottles of 90
NDC 62135-205-01 bottles of 100
NDC 62135-205-18 bottles of 180
NDC 62135-205-05 bottles of 500
10 mg
White, round scored tablet, de-bossed with "CE" over "37" on one side and functional score on the other side and supplied as:
NDC 62135-206-30 bottles of 30
NDC 62135-206-90 bottles of 90
NDC 62135-206-01 bottles of 100
NDC 62135-206-18 bottles of 180
-
STORAGE
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense contents in a tight, light-resistant container as defined in the USP with a child-resistant closure, as required.
KEEP TIGHTLY CLOSED.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
WARNING – This drug may cause toxic reaction. If such reactions occur, discontinue the drug. Constant supervision of patient is essential.
To report SUSPECTED ADVERSE REACTIONS, contact Chartwell RX, LLC. at 845-232-1683 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured by:
Chartwell Pharmaceuticals, LLC.
Congers, NY 10920
Manufactured for:
Chartwell RX, LLC.
Congers, NY 10920
L70634 Rev: 10/2021 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Methimazole Tablets, USP 5 mg - NDC 62135-205-30 - 30 Tablets Label

Methimazole Tablets, USP 5 mg - NDC 62135-205-90 - 90 Tablets Label

Methimazole Tablets, USP 5 mg - NDC 62135-205-01 - 100 Tablets Label

Methimazole Tablets, USP 5 mg - NDC 62135-205-18 - 180 Tablets Label

Methimazole Tablets, USP 5 mg - NDC 62135-205-05 - 500 Tablets Label

Methimazole Tablets, USP 10 mg - NDC 62135-206-30 - 30 Tablets Label

Methimazole Tablets, USP 10 mg - NDC 62135-206-90 - 90 Tablets Label

Methimazole Tablets, USP 10 mg - NDC 62135-206-01 - 100 Tablets Label

Methimazole Tablets, USP 10 mg - NDC 62135-206-18 - 180 Tablets Label

-
INGREDIENTS AND APPEARANCE
METHIMAZOLE
methimazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62135-205 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHIMAZOLE (UNII: 554Z48XN5E) (METHIMAZOLE - UNII:554Z48XN5E) METHIMAZOLE 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code CE;36 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62135-205-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 2 NDC:62135-205-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 3 NDC:62135-205-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 4 NDC:62135-205-18 180 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 5 NDC:62135-205-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040411 03/27/2001 METHIMAZOLE
methimazole tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:62135-206 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHIMAZOLE (UNII: 554Z48XN5E) (METHIMAZOLE - UNII:554Z48XN5E) METHIMAZOLE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code CE;37 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62135-206-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 2 NDC:62135-206-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 3 NDC:62135-206-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 4 NDC:62135-206-18 180 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040411 03/27/2001 Labeler - Chartwell RX, LLC (079394054) Registrant - Chartwell RX Sciences, LLC (081464659) Establishment Name Address ID/FEI Business Operations Chartwell Pharmaceuticals Congers, LLC 118673447 analysis(62135-205, 62135-206) , label(62135-205, 62135-206) , manufacture(62135-205, 62135-206) , pack(62135-205, 62135-206)









