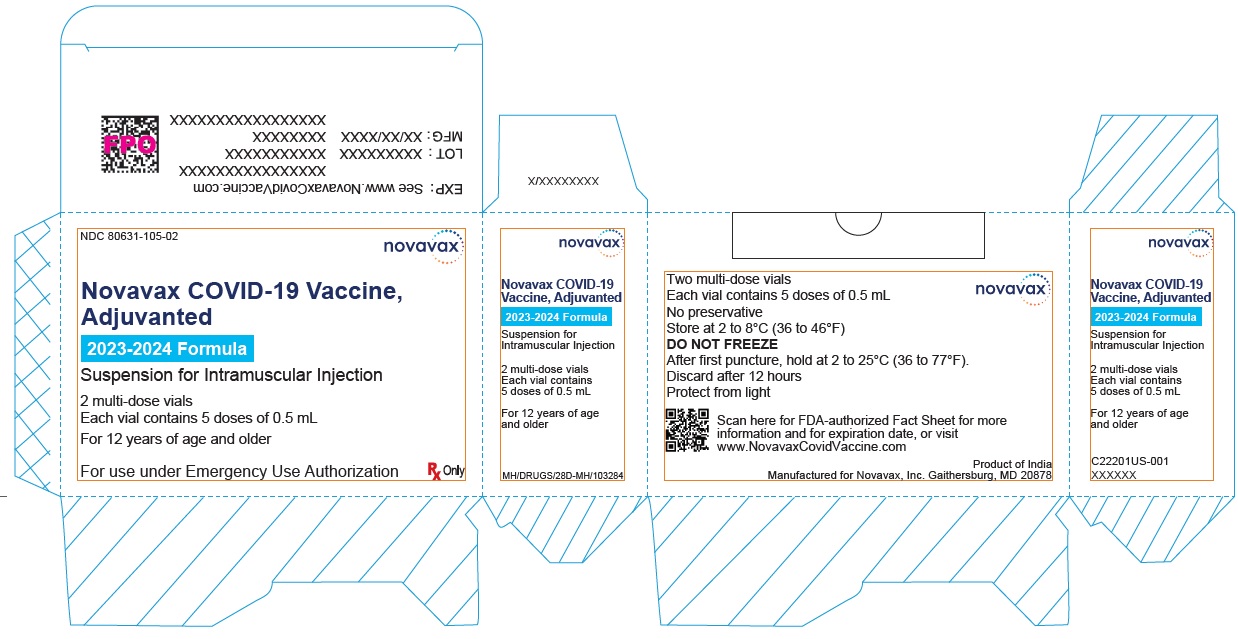
Label: NOVAVAX COVID-19 VACCINE, ADJUVANTED- nvx-cov2373 injection, suspension
-
NDC Code(s):
80631-100-01,
80631-100-10,
80631-102-01,
80631-102-10, view more80631-105-01, 80631-105-02
- Packager: Novavax, Inc.
- Category: VACCINE LABEL
Drug Label Information
Updated October 11, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE: EMERGENCY USE AUTHORIZATION OF NOVAVAX COVID-19 VACCINE, ADJUVANTED (2023-2024 FORMULA), FOR INDIVIDUALS 12 YEARS OF AGE AND OLDER HIGHLIGHTS OF EMERGENCY USE AUTHORIZATION (EUA)
These highlights do not include all the information needed to use Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) under the EUA.
See the FULL FACT SHEET FOR HEALTHCARE PROVIDERS for Novavax COVID-19 Vaccine, Adjuvanted
Novavax COVID-19 Vaccine, Adjuvanted suspension for injection, for intramuscular use.
2023-2024 Formula
Original EUA Authorized Date: 07/2022
Most Recent EUA Authorized Date: 10/2023
-----------------------RECENT MAJOR CHANGES-----------------------
Dosage and Administration, Dose and Schedule (2.3) 10/2022
-----------------------EMERGENCY USE AUTHORIZATION-----------------------
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 12 years of age and older. (1)
The Novavax COVID-19 Vaccine, Adjuvanted is not licensed for any use. (1)
See Full Fact Sheet for Healthcare Providers for the justification for emergency use of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula), information on available alternatives, and additional information on COVID-19.
-----------------------DOSAGE AND ADMINISTRATION-------------------
For intramuscular injection only. (2)
Individuals 12 Years of Age and Older Previously Vaccinated with Any COVID-19 Vaccine: administer a single 0.5 mL dose at least 2 months after receipt of the last previous dose of COVID-19 vaccine.1 (2.3)
Individuals 12 Years of Age and Older Not Previously Vaccinated with Any COVID-19 Vaccine: administer a series of two doses (0.5 mL each) 3 weeks apart. (2.3)
Individuals 12 Years of Age and Older with Certain Kinds of Immunocompromise: For individuals with certain kinds of immunocompromise2, an additional dose of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) may be administered at least 2 months following the last dose of a COVID-19 vaccine (2023-2024 Formula).3 Additional doses of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) may be administered at the discretion of the healthcare provider, taking into consideration the individual’s clinical circumstances. The timing of the additional doses may be based on the individual’s clinical circumstances. (2.3)
-------------------DOSAGE FORMS AND STRENGTHS-------------------
The Novavax COVID-19 Vaccine, Adjuvanted is a suspension for injection. A single dose is 0.5 mL. (3)
-------------------CONTRAINDICATIONS-------------------
History of a severe allergic reaction (e.g., anaphylaxis) to any component of the Novavax COVID-19 Vaccine, Adjuvanted or following a previous dose of a Novavax COVID-19 Vaccine, Adjuvanted. (4)
-------------------WARNINGS AND PRECAUTIONS-------------------
Clinical trials data provide evidence for increased risks of myocarditis and pericarditis following administration of Novavax COVID-19 Vaccine, Adjuvanted. (5.2)
-------------------ADVERSE REACTIONS-------------------
Solicited adverse reactions included: Injection site pain/tenderness, fatigue/malaise, muscle pain, headache, joint pain, nausea/vomiting, injection site redness, injection site swelling, and fever. (6)
Vaccination providers must report all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS), and cases of COVID-19 that result in hospitalization or death following administration of Novavax COVID-19 Vaccine, Adjuvanted to the Vaccine Adverse Event Reporting System (VAERS) by submitting online at https://vaers.hhs.gov/reportevent.html. For further assistance with reporting to VAERS call 1-800-822-7967. The reports should include the words “Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) EUA” in the description section of the report. To the extent feasible, report adverse events to Novavax 1-844-NOVAVAX (1-844-668-2829) or provide a copy of the VAERS form to Novavax at www.NovavaxMedInfo.com (6.3)
See FACT SHEET FOR RECIPIENTS AND CAREGIVERS.
-
SPL UNCLASSIFIED SECTION
TABLE OF CONTENTS*
2.1 Preparation for Administration
5.1 Management of Acute Allergic Reactions
5.2 Myocarditis and Pericarditis
5.5 Limitations of Vaccine Effectiveness
6.1 Clinical Trials Experience
6.3 Required Reporting for Adverse Events and Vaccine Administration Errors
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
* Sections or subsections omitted from the EUA are not listed
- SPL UNCLASSIFIED SECTION
-
1 EMERGENCY USE AUTHORIZATION
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for the emergency use of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 12 years of age and older.
Novavax COVID-19 Vaccine, Adjuvanted is not licensed for any use.
Justification for Emergency Use of Vaccines During the COVID-19 Pandemic
There is currently an outbreak of COVID-19 caused by SARS-CoV-2. The Secretary of the Department of Health and Human Services (HHS) has:
- Determined that there is a public health emergency, or a significant potential for a public health emergency related to COVID-19.4
- Declared that circumstances exist justifying the authorization of emergency use of drugs and biological products during the COVID-19 pandemic.5
An EUA is an FDA authorization for the emergency use of an unapproved product or unapproved use of an approved product (i.e., drug, biological product, or device) in the United States under certain circumstances including, but not limited to, when the Secretary of HHS declares that the use of EUA authority is justified, based on a determination that there is a public health emergency, or a significant potential for a public health emergency, that affects or has a significant potential to affect, national security or the health and security of United States citizens living abroad, and that involves biological agent(s) or a disease or condition that may be attributable to such agent(s). Criteria for issuing an EUA include:
- The biological agent(s) can cause a serious or life-threatening disease or condition;
- Based on the totality of the available scientific evidence (including data from adequate and well-controlled clinical trials, if available), it is reasonable to believe that:
- The product may be effective in diagnosing, treating, or preventing the serious or life-threatening disease or condition;
- The known and potential benefits of the product – when used to diagnose, prevent, or treat such disease or condition – outweigh the known and potential risks of the product, taking into consideration the material threat posed by the biological agent(s); and
- There is no adequate, approved, and available alternative to the product for diagnosing, preventing, or treating the serious or life-threatening disease or condition.
Information Regarding Available Alternative Vaccines for the Prevention of COVID-19 in Individuals 12 Years of Age and Older
COMIRNATY (COVID-19 Vaccine, mRNA) and SPIKEVAX (COVID-19 Vaccine, mRNA) are FDA-approved vaccines to prevent COVID-19 caused by SARS-CoV-2. There may be clinical trials of other COVID-19 vaccines, including vaccines that contain or encode the spike protein of the Omicron variant XBB.1.5 of SARS-CoV-2.
For information on clinical studies of Novavax COVID-19 Vaccine, Adjuvanted and other vaccines for the prevention of COVID-19, see www.clinicaltrials.gov.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.1 Preparation for Administration
Inspect the vial
- The Novavax COVID-19 Vaccine, Adjuvanted is a colorless to slightly yellow, clear to mildly opalescent suspension, free from visible particles.
- Gently swirl the multi-dose vial before each dose withdrawal. Do not shake.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer the vaccine if either of these conditions exist.
Prepare for administration
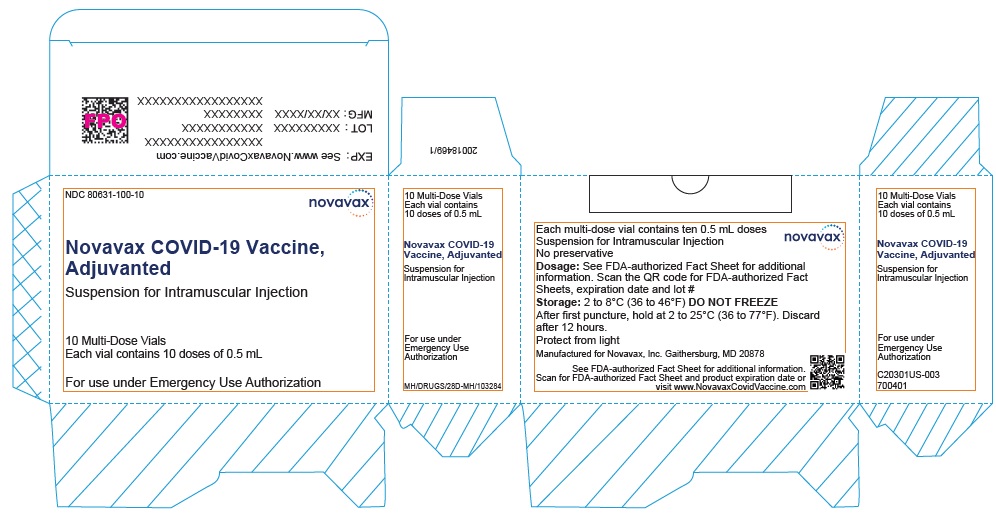
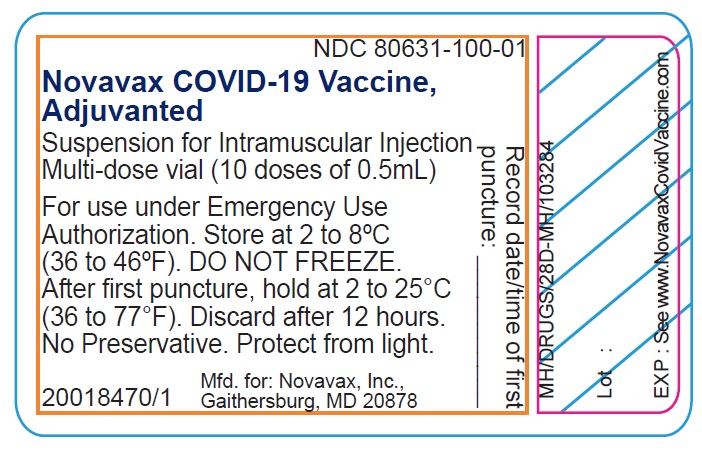
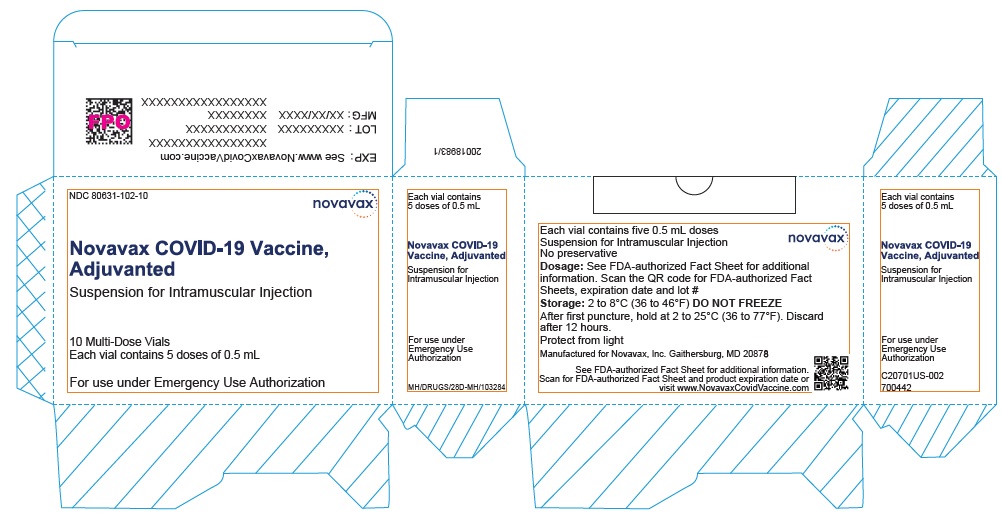
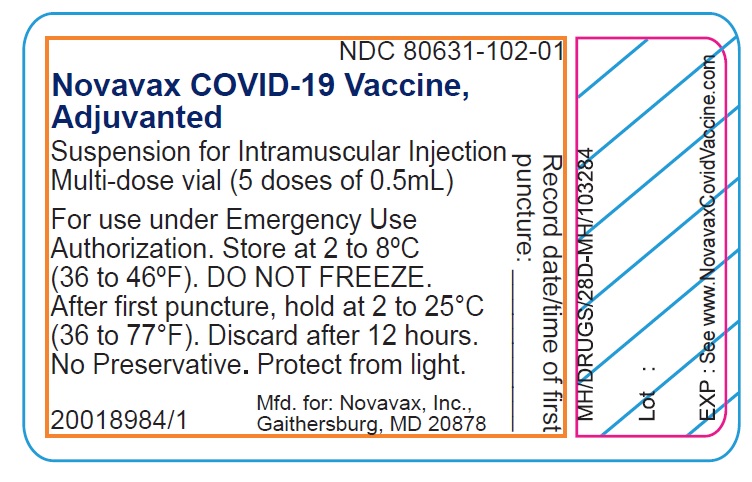
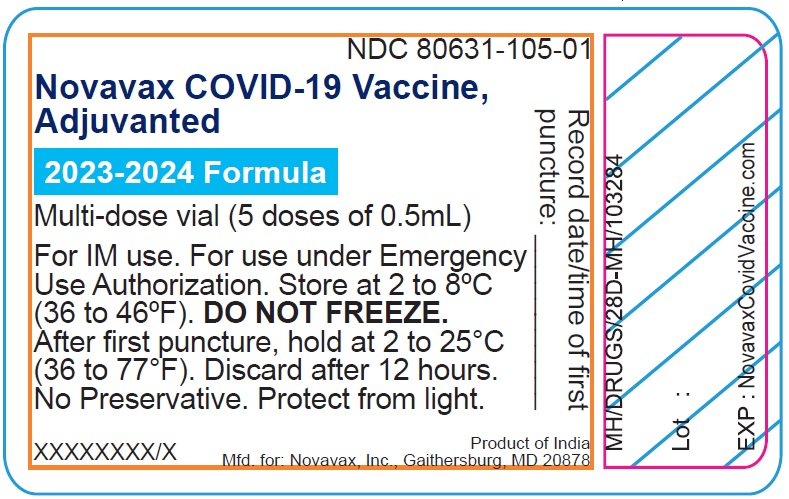
- Record the date and time of the first puncture on the vial label.
- Novavax COVID-19 Vaccine, Adjuvanted is available as multi-dose vials containing 5 doses of 0.5 mL each.
- Do not pool excess vaccine from multiple vials.
- After the first needle puncture, hold the vial between 2 to 25°C (36 to 77°F).
- Discard vial 12 hours after the first puncture.
2.3 Dose and Schedule
Individuals 12 Years of Age and Older Previously Vaccinated with Any COVID-19 Vaccine
Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) is administered intramuscularly as a single 0.5 mL dose at least 2 months after receipt of the last previous dose of COVID-19 vaccine.6
Individuals 12 Years of Age and Older Not Previously Vaccinated with Any COVID-19 Vaccine
Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) is administered intramuscularly as a series of two doses (0.5 mL each) 3 weeks apart.
Individuals 12 Years of Age and Older with Certain Kinds of Immunocompromise
For individuals with certain kinds of immunocompromise7, an additional dose of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) may be administered at least 2 months following the last dose of a COVID-19 vaccine (2023-2024 Formula).8 Additional doses of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) may be administered at the discretion of the healthcare provider, taking into consideration the individual’s clinical circumstances. The timing of the additional doses may be based on the individual’s clinical circumstances.
1 COVID-19 vaccine refers to the monovalent COVID-19 vaccines (original) and the bivalent COVID-19 vaccines (Original and Omicron BA.4/BA.5).
2 Certain kinds of immunocompromise refers to individuals who have undergone solid organ transplantation, or who are diagnosed with conditions that are considered to have an equivalent level of immunocompromise.
3 The last dose of a COVID-19 vaccine (2023-2024 Formula) refers to a dose with Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula), COMIRNATY (COVID-19 Vaccine, mRNA) (2023-2024 Formula), SPIKEVAX (COVID-19 Vaccine, mRNA) (2023-2024 Formula), Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula), or Moderna COVID-19 Vaccine (2023-2024 Formula).
4 See U.S. Department of Health and Human Services, Determination of a Public Health Emergency and Declaration that Circumstances Exist Justifying Authorizations Pursuant to Section 564(b) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3. February 4, 2020; https://www.federalregister.gov/documents/2020/02/07/2020-02496/determination-of-public-health-emergency. See also U.S. Department of Health and Human Services, Amended Determination of a Public Health Emergency or Significant Potential for a Public Health Emergency Pursuant to Section 564(b) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b). March 15, 2023 (“Amended Determination”); https://www.federalregister.gov/documents/2023/03/20/2023-05609/covid-19-emergency-use-authorization-declaration.
5 See U.S. Department of Health and Human Services, Declaration that Circumstances Exist Justifying Authorizations Pursuant to Section 564(b) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3, 85 FR 18250 (April 1, 2020); https://www.federalregister.gov/documents/2020/04/01/2020-06905/emergency-use-authorization-declaration. See also Amended Determination (“The declarations issued pursuant to section 564(b)(1) of the FD&C Act that circumstances exist justifying the authorization of emergency use of certain in vitro diagnostics, personal respiratory protective devices, other medical devices and drugs and biological products, as set forth in those declarations, and that are based on the February 4, 2020 determination, remain in effect until those declarations are terminated in accordance with section 564 of the FD&C Act.”).
6 COVID-19 vaccine refers to the monovalent COVID-19 vaccines (original) and the bivalent COVID-19 vaccines (Original and Omicron BA.4/BA.5).
7 Certain kinds of immunocompromise refers to individuals who have undergone solid organ transplantation, or who are diagnosed with conditions that are considered to have an equivalent level of immunocompromise.
8 The last dose of a COVID-19 vaccine (2023-2024 Formula) refers to a dose with Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula), COMIRNATY (COVID-19 Vaccine, mRNA) (2023-2024 Formula), SPIKEVAX (COVID-19 Vaccine, mRNA) (2023-2024 Formula), Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula), or Moderna COVID-19 Vaccine (2023-2024 Formula).
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer the Novavax COVID-19 Vaccine, Adjuvanted to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of the Novavax COVID-19 Vaccine, Adjuvanted or to individuals who had a severe allergic reaction (e.g., anaphylaxis) following a previous dose of a Novavax COVID-19 Vaccine, Adjuvanted. [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of the Novavax COVID-19 Vaccine, Adjuvanted.
Monitor Novavax COVID-19 Vaccine, Adjuvanted recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
5.2 Myocarditis and Pericarditis
Clinical trials data provide evidence for increased risks of myocarditis and pericarditis following administration of Novavax COVID-19 Vaccine, Adjuvanted [see Clinical Trials Experience (6.1)].
The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#myocarditis-pericarditis).
5.3 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines. Procedures should be in place to avoid injury from fainting.
-
6 ADVERSE REACTIONS
An overview of clinical studies contributing to the safety assessment of Novavax COVID-19 Vaccine, Adjuvanted in individuals 12 years of age and older is provided in Table 1. Participants in these clinical studies received a 2-dose initial series with a COVID-19 vaccine (referred to as a primary series) and some received one or more subsequent doses (referred to as a booster dose).
Table 1 Clinical Studies Study Age Dosing Regimens Vaccine Recipients* Abbreviation: SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. - *
- Receiving at least one dose of the intended dosing regimen.
- †
- Vaccine containing a recombinant spike protein of SARS-CoV-2 Wuhan-Hu 1 strain (Original).
- ‡
- Booster dose recipients are a subset of primary series.
- §
- Includes 39 participants who did not receive both primary series doses of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) prior to receiving a dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the booster vaccination period.
- ¶
- Participants received at least 3 doses of an mRNA COVID-19 vaccine prior to inclusion in this study.
- #
- Vaccine containing a recombinant spike protein of SARS-CoV-2 Omicron variant lineage BA.1 (Omicron BA.1), not authorized or approved in the U.S.
- Þ
- Vaccine containing a recombinant spike protein of SARS-CoV-2 Wuhan-Hu 1 strain (Original) and Omicron variant lineage BA.1 (Omicron BA.1), not authorized or approved in the U.S.
- ß
- Vaccine containing a recombinant spike protein of SARS-CoV-2 Omicron variant lineage BA.5 (Omicron BA.5), not authorized or approved in the U.S.
- à
- Vaccine containing a recombinant spike protein of SARS-CoV-2 Wuhan-Hu 1 strain (Original) and Omicron variant lineage BA.5 (Omicron BA.5), not authorized or approved in the U.S.
- è
- Restricted to participants previously vaccinated with Pfizer-BioNTech COVID-19 Vaccine.
Study 1
(NCT04611802)18 years of age and older Primary Series: 2 doses of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)† 3 weeks apart 26,106 Booster Dose: Single dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)† 12,777‡,§ 12 years through 17 years of age Primary Series: 2 doses of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)† 3 weeks apart 2,152 Booster Dose: Single dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)† 1,499‡ Study 5
(NCT05372588)18 years through 64 years (Part 1) Booster Dose: Single dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)† 274¶ Booster Dose: Single dose of monovalent Vaccine (Omicron BA.1)# 286¶ Booster Dose: Single dose of Bivalent Vaccine (Original and Omicron BA.1)Þ 269¶ 18 years of age and older (Part 2) Booster Dose: Single dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)† 251¶ Booster Dose: Single dose of monovalent Vaccine (Omicron BA.5)ß 254¶ Booster Dose: Single dose of Bivalent Vaccine (Original and Omicron BA.5)à 259¶ COV-BOOST
(ISRCTN73765130)30 years of age and older Booster Dose: Single dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)† 114è The safety data accrued with the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) [no longer authorized for use in the U.S.] and from Novavax’s adjuvanted monovalent COVID-19 vaccine (Omicron BA.1) [not authorized or approved in the U.S., hereafter referred to as monovalent vaccine (Omicron BA.1)], Novavax’s adjuvanted monovalent COVID-19 vaccine (Omicron BA.5) [not authorized or approved in the U.S., hereafter referred to as monovalent vaccine (Omicron BA.5)], Novavax’s adjuvanted bivalent vaccine (Original and Omicron BA.1) [not authorized or approved in the U.S, hereafter referred to as bivalent vaccine (Original and Omicron BA.1)] and Novavax’s adjuvanted bivalent vaccine (Original and Omicron BA.5) [not authorized or approved in the U.S., hereafter referred to as bivalent vaccine (Original and Omicron BA.5)] are relevant to Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) because these vaccines are manufactured using a similar process.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) Administered as a Two-Dose Primary Series
Participants 18 years of age and older
Safety of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) was assessed in a clinical study conducted in the United States (US) and Mexico (NCT04611802; Study 1). In this study, 26,106 participants 18 years of age and older have received at least one dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent).
Adolescents 12 Through 17 Years of Age
Safety of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in adolescents was assessed in the adolescent primary series expansion of Study 1 conducted in the US. In this study, 2,232 participants 12 through 17 years of age have received at least one dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (n=1,487) or placebo (n=745).
Safety Data from Study 1
In Study 1, an ongoing Phase 3, multicenter, randomized, observer-blinded, placebo-controlled study, participants 18 years of age and older have received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (n=19,735) or placebo (n=9,847). Overall, 52.0% were male, 48.0% were female; 75.0% were White, 11.8% were Black or African American, 4.1% were Asian, 6.7% were American Indian (including Native Americans) or Alaskan Native, and 1.6% were multiple races; 21.9% were Hispanic/Latino. Demographic characteristics of participants were well balanced between the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and placebo groups. During the study, COVID-19 vaccines authorized for emergency use became available, and participants, when eligible for vaccination, were offered the opportunity to cross over from the originally assigned study treatment to the other study treatment (vaccine or placebo) in a blinded fashion (“blinded crossover”). In the post-crossover period, 6,416 participants received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), and 15,298 participants received placebo. The demographic characteristics of participants in the pre- and post-crossover groups were comparable. Due to data quality issues at two study sites, a total of 289 additional participants were excluded from the safety analysis set.
Study 1 also included an adolescent primary series expansion. In the pre-crossover period, among adolescent participants who received at least one dose of vaccine (n=1487) or placebo (n=745), 52.5% were male, 47.5% were female; 74.4% were White, 13.9% were Black or African American, 3.4% were Asian, 2.1% were American Indian (including Native Americans) or Alaskan Native, and 5.3% were multiple races; 18.5% were Hispanic/Latino. Demographic characteristics were well balanced between the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and placebo groups. During the study, COVID-19 vaccines authorized for emergency use became available, and participants, when eligible for vaccination, were offered the opportunity to cross over from the originally assigned study treatment to the other study treatment (vaccine or placebo) in a blinded fashion (“blinded crossover”). In the post-crossover period, 665 participants received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), and 1,353 participants received placebo. The demographic characteristics of participants in the pre- and post-crossover groups were comparable.
Study 1 was amended to include a booster dose in which 12,738 individuals 18 to 96 years of age received a booster dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) starting approximately 6 months after the two-dose primary series.
Participants 18 years of age and older
Solicited Adverse Reactions
During the pre-crossover period, local and systemic adverse reactions were solicited within 7 days following each dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) or placebo in participants using an electronic diary.
The reported frequency and severity of solicited local and systemic adverse reactions series are presented for participants 18 through 64 years of age in Table 2.
Table 2 Number and Percentage of Participants with Solicited Local and Systemic Adverse Reactions Starting within 7 Days* After Each Dose in Participants 18 Years through 64 Years of Age (Solicited Safety Set,† Dose 1 and Dose 2)‡ Event Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) Placebo§ Primary Series Primary Series Dose 1
N = 15884
n (%)Dose 2
N = 15148
n (%)Dose 1
N = 7868
n (%)Dose 2
N = 7361
n (%)- *
- 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (eDiary).
- †
- Solicited safety set includes participants who received at least one dose of study vaccine and completed their eDiary.
- ‡
- Absence of rows for Grade 4 adverse reactions indicates no events were reported.
- §
- Placebo was a saline solution.
- ¶
- Grade 3 pain: Defined as any use of narcotic pain reliever or prevents daily activity.
- #
- Grade 3 tenderness: Defined as significant discomfort at rest.
- Þ
- Grade 4 pain/ tenderness: Defined as Emergency Room (ER) visit or hospitalization.
- ß
- Grade 3 redness (erythema): Defined as > 10 cm.
- à
- Grade 3 swelling: Defined as > 10 cm or prevents daily activity.
- è
- Grade 3 fever: Defined as 39.0 to 40°C (102.1 to 104°F).
- ð
- Grade 4 fever: Defined as > 40°C (> 104°F).
- ø
- Grade 3 headache: Defined as significant; any use of narcotic pain reliever or prevents daily activity.
- ý
- Grade 4 headache, fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as ER visit or hospitalization.
- £
- Grade 3 fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as significant; prevents daily activity.
- ¥
- Grade 3 nausea or vomiting: Defined as prevents daily activity, requires outpatient IV hydration.
- Œ
- Grade 4 nausea or vomiting: Defined as ER visit or hospitalization for hypotensive shock.
Local Adverse Reactions Pain/tenderness Any Grade 9604 (60.5) 12234 (80.8) 1706 (21.7) 1623 (22.0) Grade 3¶,# 174 (1.1) 951 (6.3) 17 (0.2) 20 (0.3) Grade 4Þ 0 5 (0.03) 0 1 (0.01) Redness (erythema) Any Grade 151 (1.0) 1040 (6.9) 21 (0.3) 26 (0.4) Grade 3ß 3 (0.02) 134 (0.9) 0 2 (0.03) Swelling Any Grade 137 (0.9) 943 (6.2) 24 (0.3) 22 (0.3) Grade 3à 7 (0.04) 82 (0.5) 3 (0.04) 1 (0.01) Systemic Adverse Reactions Fever Any Grade 56 (0.4) 941 (6.2) 31 (0.4) 16 (0.2) Grade 3è 7 (0.04) 60 (0.4) 7 (0.09) 2 (0.03) Grade 4ð 4 (0.03) 2 (0.01) 1 (0.01) 0 Headache Any Grade
4158 (26.2) 7128 (47.1) 1866 (23.7) 1492 (20.3) Grade 3ø 132 (0.8) 492 (3.2) 58 (0.7) 36 (0.5) Grade 4ý 4 (0.03) 5 (0.03) 1 (0.01) 2 (0.03) Fatigue/malaise Any Grade 4892 (30.8) 8825 (58.3) 2095 (26.6) 1889 (25.7) Grade 3£ 249 (1.6) 1591 (10.5) 113 (1.4) 114 (1.5) Grade 4ý 8 (0.05) 7 (0.05) 1 (0.01) 3 (0.04) Muscle pain (myalgia) Any Grade 3827 (24.1) 7682 (50.7) 1073 (13.6) 900 (12.2) Grade 3£ 79 (0.5) 805 (5.3) 31 (0.4) 28 (0.4) Grade 4ý 2 (0.01) 5 (0.03) 1 (0.01) 4 (0.05) Joint pain (arthralgia) Any Grade 1260 (7.9) 3542 (23.4) 522 (6.6) 504 (6.8) Grade 3£ 49 (0.3) 393 (2.6) 25 (0.3) 22 (0.3) Grade 4ý 1 (< 0.01) 5 (0.03) 0 2 (0.03) Nausea or vomiting Any Grade 1069 (6.7) 1822 (12.0) 466 (5.9) 417 (5.7) Grade 3¥ 18 (0.1) 26 (0.2) 7 (0.09) 7 (0.1) Grade 4Œ 4 (0.03) 7 (0.05) 2 (0.03) 2 (0.03) The reported frequency and severity of solicited local and systemic adverse reactions are presented for participants 65 years of age and older in Table 3.
Table 3 Number and Percentage of Participants with Solicited Local and Systemic Adverse Reactions Starting within 7 Days* After Each Dose in Participants 65 Years of Age and Older (Solicited Safety Set,† Dose 1 and Dose 2)‡ Event Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) Placebo§ Primary Series Primary Series Dose 1
N = 2251
n (%)Dose 2
N = 2048
n (%)Dose 1
N = 1114
n (%)Dose 2
N = 978
n (%)- *
- 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (eDiary).
- †
- Solicited safety set includes participants who received at least one dose of study vaccine and completed their eDiary.
- ‡
- Absence of rows for Grade 4 adverse reactions indicates no events were reported.
- §
- Placebo was a saline solution.
- ¶
- Grade 3 pain: Defined as any use of narcotic pain reliever or prevents daily activity.
- #
- Grade 3 tenderness: Defined as significant discomfort at rest.
- Þ
- Grade 3 redness (erythema): Defined as > 10 cm.
- ß
- Grade 3 swelling: Defined as > 10 cm or prevents daily activity.
- à
- Grade 3 fever: Defined as 39.0 to 40°C (102.1 to 104°F).
- è
- Grade 3 headache: Defined as significant; any use of narcotic pain reliever or prevents daily activity.
- ð
- Grade 4 headache, joint pain (arthralgia): Defined as ER visit or hospitalization.
- ø
- Grade 3 fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as significant; prevents daily activity.
- ý
- Grade 3 nausea or vomiting: Defined as prevents daily activity, requires outpatient IV hydration.
Local Adverse Reactions Pain/tenderness Any Grade 854 (37.9) 1258 (61.4) 175 (15.7) 161 (16.5) Grade 3¶,# 13 (0.6) 43 (2.1) 3 (0.3) 1 (0.1) Redness (erythema) Any Grade 16 (0.7) 99 (4.8) 5 (0.4) 4 (0.4) Grade 3Þ 0 7 (0.3) 0 0 Swelling Any Grade 18 (0.8) 111 (5.4) 1 (0.09) 4 (0.4) Grade 3ß 1 (0.04) 8 (0.4) 0 1 (0.1) Systemic Adverse Reactions Fever Any Grade 8 (0.4) 40 (2.0) 3 (0.3) 7 (0.7) Grade 3à 1 (0.04) 2 (0.1) 0 1 (0.1) Headache Any Grade 344 (15.3) 502 (24.5) 184 (16.5) 144 (14.7) Grade 3è 12 (0.5) 18 (0.9) 4 (0.4) 2 (0.2) Grade 4ð 1 (0.04) 1 (0.05) 0 0 Fatigue/malaise Any Grade 444 (19.7) 714 (34.9) 202 (18.1) 182 (18.6) Grade 3ø 23 (1.0) 68 (3.3) 5 (0.4) 13 (1.3) Muscle pain (myalgia) Any Grade 284 (12.6) 561 (27.4) 125 (11.2) 102 (10.4) Grade 3ø 3 (0.1) 32 (1.6) 4 (0.4) 2 (0.2) Joint pain (arthralgia) Any Grade 139 (6.2) 271 (13.2) 71 (6.4) 63 (6.4) Grade 3ø 4 (0.2) 16 (0.8) 4 (0.4) 2 (0.2) Grade 4ð 0 1 (0.05) 0 0 Nausea/vomiting Any Grade 81 (3.6) 108 (5.3) 32 (2.9) 35 (3.6) Grade 3ý 0 3 (0.1) 0 0 Unsolicited Adverse Events (non-serious and serious)
In Study 1, participants were monitored for non-serious unsolicited adverse events from the first dose through 28 days after the second dose in both the pre- and post-crossover periods and for serious adverse events for the duration of study participation. Participants who only received the first dose in the pre- and post-crossover periods were monitored for non-serious unsolicited adverse events through 49 days after administration of vaccine or placebo and for serious adverse events for the duration of study participation. In the pre-crossover period 19,735 participants received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and 9,847 participants received placebo. In the post-crossover period, 6,416 participants received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and 15,298 received placebo. Of participants who received two doses of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the pre-crossover period (n=19,111), 78% had a follow-up duration of at least 2 months (median = 2.5 months) after Dose 2. Of participants who received two doses of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the post-crossover period (n=6,346), 99% had a follow-up duration of at least 2 months (median = 4.4 months) after the last dose.
From Dose 1 through 28 days following Dose 2 in the pre-crossover period, the overall frequency of non-serious unsolicited adverse events was similar in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group (11.6%) and the placebo group (11.2%). The most frequently reported unsolicited adverse reactions were chills (0.4% vaccine recipients vs. 0.1% placebo recipients), lymphadenopathy-related reactions (0.3% vaccine recipients vs. 0.1% placebo recipients), and injection site pruritus (0.1% vaccine recipients vs. 0.0% placebo recipients). Lymphadenopathy-related reactions included lymphadenopathy, lymphadenitis, lymph node pain, and axillary pain. All lymphadenopathy-related reactions occurred in participants 18 through 64 years of age.
In the pre-crossover period, serious adverse events were reported by 199 (1.0%) participants in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group and by 108 (1.1%) participants in the placebo group. In the post-crossover period, serious adverse events were reported by 88 (1.4%) participants who received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and by 178 (1.2%) participants who received placebo.
Within 7 days of any dose (including 26,151 Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) recipients and 25,145 placebo recipients in both the pre- and post-crossover periods), hypersensitivity reactions (including urticaria, hypersensitivity, angioedema, and swelling of the face, lips, ear, and/or eyelids) were reported by 26 participants after the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (0.1%) and 8 participants after placebo (0.03%). Of these events, 1 reaction (generalized urticaria and facial angioedema with a duration of 2 days) was serious and occurred 2 days after Dose 1 of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent).
Within 28 days of any dose, the following numerical imbalances with more events in vaccine than placebo recipients (including 26,151 Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) recipients and 25,145 placebo recipients in both the pre- and post-crossover periods) were observed for the following serious and other adverse events of interest.
- Myocarditis and/or pericarditis were reported by two participants after the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (0.01%) and no participants after placebo. One serious event was reported by a 67-year-old male 28 days after Dose 1, associated with concomitant COVID-19, and one non-serious event was reported by a 20-year-old male 10 days after Dose 1. Among the two reported events, one was reported as resolved and one did not have follow-up available. Reports of myocarditis and/or pericarditis from Study 1 and Study 2 provide evidence for increased risks of myocarditis and pericarditis following administration of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent).
- Events of cardiomyopathy or cardiac failure were reported by eight participants after the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (0.03%) and one participant after placebo (< 0.01%). All events were serious. Additionally, an event of congestive cardiac failure was reported after the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) by a participant who was excluded from the safety analysis set. Currently available information on cardiomyopathy or cardiac failure is insufficient to determine a causal relationship with the vaccine.
- Events of acute cholecystitis were reported by six participants after the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (0.02%) and two participants after placebo (0.01%). All events were serious. Currently available information on cholecystitis is insufficient to determine a causal relationship with the vaccine.
- A total of 12 non-cardiac, non-neurovascular thrombotic and embolic events were reported by 11 participants after the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (0.04%) and a total of seven events were reported by six participants after placebo (0.02%). Events following the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) included pulmonary embolism (n=5), deep vein thrombosis (n=2), thrombosis (n=2), and portal vein thrombosis, mesenteric artery thrombosis, and peripheral arterial occlusive disease (n=1 each); six of the events were serious, including pulmonary embolism (n=5) and deep vein thrombosis (n=1). Events following placebo included pulmonary embolism (n=3), and deep vein thrombosis and peripheral arterial occlusive disease (n=2 each), all of which were serious except deep vein thrombosis and peripheral arterial occlusive disease (n=1 each). Currently available information on non-cardiac, non-neurovascular thrombotic and embolic events is insufficient to determine a causal relationship with the vaccine.
Events of uveitis (iritis, uveitis, iridocyclitis) were reported by 3 participants after Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (0.01%) and 2 participants after placebo (0.01%). All events were non-serious. One participant had onset of uveitis after Dose 1 of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) which resolved and then recurred following Dose 2. The two placebo recipients with events appeared to have had a previous history of uveitis and one of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) recipients had a history of iritis. Currently available information on uveitis is insufficient to determine a causal relationship with the vaccine.
Adolescents 12 Through 17 Years of Age
Solicited Adverse Reactions
During the pre-crossover period, local and systemic adverse reactions were solicited within 7 days following each dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) or placebo in participants using an electronic diary.
The reported frequency and severity of solicited local and systemic adverse reactions are presented for participants 12 through 17 years of age in Table 4.
Table 4 Number and Percentage of Participants with Solicited Local and Systemic Adverse Reactions Starting within 7 Days* After Each Dose in Participants 12 Years through 17 Years of Age (Solicited Safety Set,† Dose 1 and Dose 2)‡ Event Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) Placebo§ Dose 1
N = 1448
n (%)Dose 2
N = 1394
n (%)Dose 1
N = 726
n (%)Dose 2
N = 686
n (%)- *
- 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (eDiary).
- †
- Solicited safety set includes participants who received at least one dose of study vaccine and completed their eDiary.
- ‡
- Absence of rows for Grade 4 adverse reactions indicates no events were reported.
- §
- Placebo was a saline solution.
- ¶
- Grade 3 pain: Defined as any use of narcotic pain reliever or prevents daily activity.
- #
- Grade 3 tenderness: Defined as significant discomfort at rest.
- Þ
- Grade 3 redness (erythema): Defined as > 10 cm.
- ß
- Grade 3 swelling: Defined as > 10 cm or prevents daily activity.
- à
- Grade 3 fever: Defined as 39.0 to 40°C (102.1 to 104°F).
- è
- Grade 4 fever: Defined as > 40°C (> 104°F).
- ð
- Grade 3 headache: Defined as significant; any use of narcotic pain reliever or prevents daily activity.
- ø
- Grade 4 headache, fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as ER visit or hospitalization.
- ý
- Grade 3 fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as significant; prevents daily activity.
- £
- Grade 3 nausea or vomiting: Defined as prevents daily activity, requires outpatient IV hydration.
- ¥
- Grade 4 nausea or vomiting: Defined as ER visit or hospitalization for hypotensive shock.
Local Adverse Reactions Pain/tenderness Any Grade 945 (65.3) 1045 (75.0) 204 (28.1) 141 (20.6) Grade 3¶,# 22 (1.5) 108 (7.7) 4 (0.6) 4 (0.6) Redness (erythema) Any Grade 15 (1.0) 104 (7.5) 5 (0.7) 0 Grade 3Þ 0 10 (0.7) 0 0 Swelling Any Grade 20 (1.4) 111 (8.0) 3 (0.4) 1 (0.1) Grade 3ß 0 8 (0.6) 1 (0.1) 0 Systemic Adverse Reactions Fever Any Grade 11 (0.8) 235 (16.9) 5 (0.7) 1 (0.1) Grade 3à 1 (0.07) 31 (2.2) 0 0 Grade 4è 2 (0.1) 0 0 0 Headache Any Grade 440 (30.4) 793 (56.9) 181 (24.9) 119 (17.3) Grade 3ð 13 (0.9) 87 (6.2) 12 (1.7) 14 (2.0) Grade 4ø 0 1 (0.07) 0 0 Fatigue/malaise Any Grade 418 (28.9) 807 (57.9) 142 (19.6) 113 (16.5) Grade 3ý 33 (2.3) 223 (16.0) 13 (1.8) 13 (1.9) Muscle pain (myalgia) Any Grade 492 (34.0) 683 (49.0) 114 (15.7) 82 (12.0) Grade 3ý 17 (1.2) 104 (7.5) 4 (0.6) 6 (0.9) Joint pain (arthralgia) Any Grade 102 (7.0) 226 (16.2) 35 (4.8) 21 (3.1) Grade 3ý 6 (0.4) 40 (2.9) 1 (0.1) 2 (0.3) Nausea or vomiting Any Grade 113 (7.8) 277 (19.9) 56 (7.7) 33 (4.8) Grade 3£ 2 (0.1) 14 (1.0) 3 (0.4) 3 (0.4) Grade 4¥ 0 1 (0.07) 0 0 Unsolicited Adverse Events (non-serious and serious)
In Study 1, participants were monitored for non-serious unsolicited adverse events from the first dose through 28 days after the second dose in both the pre- and post-crossover periods and for serious adverse events for the duration of study participation. Participants who only received the first dose in the pre- and post-crossover periods were monitored for non-serious unsolicited adverse events through 49 days after administration of vaccine or placebo and for serious adverse events for the duration of study participation. In the pre-crossover period 1,487 participants received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and 745 participants received placebo. In the post-crossover period, 665 participants received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and 1,353 received placebo. Of participants who received two doses of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the pre-crossover period (n=1,468), 86% had a follow-up duration of at least 2 months (median = 71 days) after Dose 2. Of participants who received two doses of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the post-crossover period (n=638), 43% had a follow-up duration of at least 1 month (median = 30 days) after the last dose.
From Dose 1 through 28 days following Dose 2 in the pre-crossover period, the overall frequency of non-serious unsolicited adverse events was similar in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group (15.5%) and the placebo group (15.3%). The most frequently reported unsolicited adverse reactions were lymphadenopathy-related reactions (0.9% vaccine recipients vs. 0.0% placebo recipients), fatigue (0.5% vaccine recipients vs. 0.0% placebo recipients), decreased appetite (0.3% vaccine recipients vs. 0.0% placebo recipients), arthralgia (0.2% vaccine recipients vs. 0.0% placebo recipients), injection site pruritus (0.2% vaccine recipients vs. 0.0% placebo recipients), and myalgia (0.1% vaccine recipients vs. 0.0% placebo recipients). Lymphadenopathy-related reactions included lymphadenopathy, lymph node pain, and axillary pain.
In the pre-crossover period, serious adverse events were reported by 7 (0.5%) participants in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group and by 2 (0.3%) participants in the placebo group. In the post-crossover period, serious adverse events were reported by 3 (0.5%) participants who received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and by 2 (0.1%) participants who received placebo.
Within 28 days of any dose, one serious adverse event of interest of myocarditis was observed. The event was reported by a 16-year-old adolescent participant 2 days after Dose 2 of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent).
Safety Data from Other Studies with Primary Series
Study 2 was a randomized, placebo-controlled study that included a crossover design. Approximately 10,800 participants received at least one dose of a COVID-19 vaccine containing SARS-CoV-2 recombinant spike (rS) protein and Matrix-M adjuvant, manufactured by a different process than the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) evaluated in Study 1, and approximately 10,900 participants received at least one dose of placebo.
Serious events of myocarditis in a 19-year-old male and pericarditis in a 60-year-old female were reported within 10 days following administration of Dose 2 and Dose 1, respectively, of the vaccine. Both events were reported as resolved. No events of myocarditis or pericarditis were reported following administration of placebo.
A serious event of Guillain Barré syndrome was reported 9 days following administration of Dose 1 of the vaccine. No events of Guillain Barré syndrome were reported following administration of placebo.
In Studies 3 and 4, approximately 5,500 participants received at least one dose of a COVID-19 vaccine containing SARS-CoV-2 recombinant spike (rS) protein and Matrix-M adjuvant, manufactured by a different process than the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) evaluated in Study 1. No serious adverse events considered related to vaccination were reported in these studies. No events of myocarditis/pericarditis or Guillain Barré syndrome were reported in vaccine recipients in these studies.
Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) Administered as a Booster Dose Following a Primary Series of Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) in Participants 18 Years or Older
In an open label portion of Study 1, 12,738 participants 18 years of age and older (based on enrollment until March 26, 2022) received a single booster dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (0.5 mL) at least 6 months after the two-dose primary series (median of 11.0 months between completion of primary series and booster dose). Safety analyses included evaluation of solicited local and systemic adverse reactions within 7 days after a booster dose (n=238) and nonserious unsolicited adverse events within 28 days after a booster dose (n=298). Safety analysis also included evaluation of serious adverse events and adverse events of interest after a booster dose (n=12,738) with a median follow-up of 121 days post booster dose through data extraction of August 18, 2022. The safety follow-up is ongoing.
Among the 12,738 boosted participants, 84.3% were between 18 and 64 years of age and 15.7% were 65 years of age and older, 50.6% were male, 49.4% were female; 72.6% were White, 14.4% were Black or African American, 3.8% were Asian, 6.5% were American Indian (including Native Americans) or Alaskan Native, 0.2% were Native Hawaiian or Other Pacific Islander, and 1.7% were multiple races; 21.4% were Hispanic or Latino.
Solicited Adverse Reactions
Local and systemic adverse reactions were solicited within 7 days following the third (booster) dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) using an electronic diary.
The reported frequency and severity of solicited local and systemic adverse reactions in participants 18 years of age and older are presented in Table 5.
Table 5 Number and Percentage of Participants with Solicited Local and Systemic Adverse Reactions Starting within 7* Days After Booster Dose in Participants 18 Years of Age and Older (Booster Safety Analysis Set †) ‡ Event Novavax COVID-19 Vaccine,
Adjuvanted (Original monovalent)
N = 238
n (%)- *
- 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (eDiary).
- †
- The analysis included a total of 238 participants who received the booster dose who completed their eDiary
- ‡
- Absence of rows for Grade 4 adverse reactions indicates no events were reported.
- §
- Grade 3 pain: Defined as any use of narcotic pain reliever or prevents daily activity.
- ¶
- Grade 3 tenderness: Defined as significant discomfort at rest.
- #
- Grade 3 redness (erythema): Defined as > 10 cm.
- Þ
- Grade 3 swelling: Defined as > 10 cm or prevents daily activity.
- ß
- Grade 3 fever: Defined as 39.0 to 40°C (102.1 to 104°F).
- à
- Grade 3 headache: Defined as significant; any use of narcotic pain reliever or prevents daily activity.
- è
- Grade 3 fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as significant; prevents daily activity.
- ð
- Grade 4 fatigue/malaise, muscle pain (myalgia): Defined as ER visit or hospitalization.
- ø
- Grade 3 nausea or vomiting: Defined as prevents daily activity, requires outpatient IV hydration.
- ý
- Grade 4 nausea or vomiting: Defined as ER visit or hospitalization for hypotensive shock.
Local Adverse Reactions Pain/tenderness Any Grade 193 (81.1) Grade 3§,¶ 18 (7.6) Redness (erythema) Any Grade 15 (6.3) Grade 3# 1 (0.4) Swelling Any Grade 20 (8.4) Grade 3Þ 2 (0.8) Systemic Adverse Reactions Fever Any Grade 15 (6.3) Grade 3ß 2 (0.8) Headache Any Grade 126 (52.9) Grade 3à 14 (5.9) Fatigue/malaise Any Grade 151 (63.4) Grade 3è 41 (17.2) Grade 4ð 2 (0.8) Muscle pain (myalgia) Any Grade 150 (63.0) Grade 3è 20 (8.4) Grade 4ð 2 (0.8) Joint pain (arthralgia) Any Grade 72 (30.3) Grade 3è 9 (3.8) Nausea or vomiting Any Grade 35 (14.7) Grade 3ø 2 (0.8) Grade 4ý 1 (0.4) Unsolicited Adverse Events (non-serious and serious)
Participants were monitored through 28 days after the booster dose for unsolicited adverse events. Out of 12,738 total booster participants, data are available for 298 participants for non-serious unsolicited adverse events until May 19, 2022 (median follow-up post booster of 122 days). There were no unsolicited adverse events that occurred in more than one participant.
Additionally, data for serious adverse events and adverse events of interest, including but not limited to allergic, neurologic, inflammatory, vascular, and autoimmune disorders, are available for 12,738 participants until August 18, 2022 (median follow-up post booster of 121 days).
An event of myocarditis was reported by a 28-year-old male participant 3 days after a booster dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in Study 1. The event following the booster dose was adjudicated as a non-ST elevation myocardial infarction; however, clinical features were also consistent with myocarditis (chest pain and elevated troponin), and no cardiac catheterization or cardiac MRI was performed during the acute presentation.
A serious adverse event of autoimmune hepatitis was reported in a 57-year-old male participant approximately 12 days after a booster dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent). A year prior to vaccination, the participant had transient increases in alanine transferase (ALT), up to 3 times the upper limit of normal (ULN). From a normal baseline ALT prior to receipt of the first dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), ALT increased to 4 times ULN following the second dose of the primary series. After the booster dose, a recurrent and higher ALT increase was observed (7 times ULN). Viral hepatitis tests were negative, and no alternative etiologies have been identified. The event has been ongoing for 8 months and is not resolved with azathioprine treatment. Currently available information for this event is insufficient to determine a causal relationship with the vaccine.
Two serious adverse events in the injected arm were reported, including muscle edema in a 51-year-old female with onset 7 days after booster vaccination and cellulitis of the injection site in a 58-year-old male with onset 3 days after booster vaccination. The cellulitis resolved following antibiotic and steroid treatment. The muscle edema was not responsive to non-steroidal anti-inflammatory agents and has been ongoing for 6 months and is not resolved. Available information for these events is insufficient to determine a causal relationship with the vaccine.
A serious adverse event of extensive left leg and pelvic deep vein thrombosis and pulmonary embolism was reported 7 and 10 days, respectively, post booster in a 35-year-old female participant receiving oral contraceptive therapy. She required surgical intervention, thrombolytic therapy, and needs prolonged anti-coagulation. Available information for these events is insufficient to determine a causal relationship with the vaccine.
Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent), Monovalent Vaccine (Omicron BA.1), Bivalent Vaccine (Original and Omicron BA.1) Administered as a Booster Dose Following Primary and Booster Vaccination with Another Authorized or Approved COVID-19 Vaccine in Individuals 18 through 64 Years of Age
The safety of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), the monovalent vaccine (Omicron BA.1) and the bivalent vaccine (Original and Omicron BA.1) administered as a booster dose to individuals 18 through 64 years of age, previously vaccinated with three doses of an authorized or approved mRNA COVID-19 vaccine was assessed in a randomized, observer blind study (NCT05372588, Part 1 in Australia; Study 5).
The safety analysis set included 274 participants in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group, 286 participants in the monovalent vaccine (Omicron BA.1) group, and 269 participants in the bivalent vaccine (Original and Omicron BA.1) group. The median time since the last COVID-19 vaccination was 180.0 days. The median age of the population was 41 years (range 18-64); 727 (87.7%) participants were 18 through 54 years of age and 102 (12.3%) were 55 years and older. Overall, 46.1% were male, 53.9% were female, 2.4% were Hispanic or Latino, 80.6% were White, 0.2% were African American, 0.6% were Aboriginal Australian, 14.6% were Asian, 0.2% were Native Hawaiian or Pacific Islander, 2.7% were other races, and 1.1% were Multiracial. Demographic characteristics were similar across the three groups. Safety analysis included a median follow-up of 66 days post booster dose through data cutoff date of 01 September 2022. The safety follow-up is ongoing.
Solicited Adverse Reactions
Local and systemic adverse reactions were solicited within 7 days following vaccination with Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), the monovalent vaccine (Omicron BA.1), or the bivalent vaccine (Original and Omicron BA.1) using an electronic diary. The reported frequency and severity of solicited local and systemic adverse reactions are presented for participants 18 to 64 years of age in Table 6.
Table 6 Number and Percentage of Participants with Solicited Local and Systemic Adverse Reactions Starting within 7* Days After Booster Dose in Participants 18 Years through 64 Years of Age Who Received Primary and Booster Vaccination with Another Authorized or Approved COVID-19 Vaccine (Safety Analysis Set)† Event Monovalent Vaccine
(Omicron BA.1)
N = 283Novavax COVID-19
Vaccine (Original monovalent)
N = 272Bivalent Vaccine
(Original and
Omicron BA.1)
N = 268- *
- 7 days included day of vaccination and the subsequent 6 days. Events were collected in the electronic diary (eDiary). The analysis included a total of 823 participants who received the booster dose who completed their eDiary.
- †
- Absence of rows for Grade 3 or Grade 4 adverse reactions indicates no events were reported.
- ‡
- Grade 3 pain: Defined as any use of narcotic pain reliever or prevents daily activity.
- §
- Grade 3 tenderness: Defined as significant discomfort at rest.
- ¶
- Grade 3 redness (erythema): Defined as > 10 cm.
- #
- Grade 3 fever: Defined as 39.0 to 40°C (102.1 to 104°F). Grade 4 fever: Defined as >40°C (>104°F).
- Þ
- Grade 3 headache: Defined as significant; any use of narcotic pain reliever or prevents daily activity.
- ß
- Grade 3 fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as significant; prevents daily activity.
- à
- Grade 3 nausea or vomiting: Defined as prevents daily activity or requires outpatient IV hydration.
Local Adverse Reactions Pain/tenderness Any Grade 196 (69.3) 192 (70.6) 173 (64.6) Grade 3‡,§ 5 (1.8) 1 (0.4) 2 (0.7) Redness (erythema) Any Grade 7 (2.5) 3 (1.1) 3 (1.1) Grade 3¶ 0 0 1 (0.4) Swelling Any Grade 7 (2.5) 3 (1.1) 4 (1.5) Systemic Adverse Reactions Fever Any Grade 5 (1.8) 2 (0.7) 1 (0.4) Grade 3# 1 (0.4) 0 0 Grade 4# 1 (0.4) 0 0 Headache Any Grade 106 (37.5) 95 (34.9) 96 (35.8) Grade 3Þ 1 (0.4) 3 (1.1) 1 (0.4) Fatigue/malaise Any Grade 127 (44.9) 111 (40.8) 121 (45.1) Grade 3ß 15 (5.3) 8 (2.9) 7 (2.6) Muscle pain (myalgia) Any Grade 71 (25.1) 66 (24.3) 64 (23.9) Grade 3ß 5 (1.8) 0 0 Joint pain (arthralgia) Any Grade 27 (9.5) 29 (10.7) 16 (6.0) Grade 3ß 2 (0.7) 0 1 (0.4) Nausea or vomiting Any Grade 21 (7.4) 19 (7.0) 23 (8.6) Grade 3à 0 1 (0.4) 0 Unsolicited Adverse Events (non-serious and serious)
Participants were monitored through 36 days after the booster dose for unsolicited adverse events. Additionally, data for serious adverse events and adverse events of interest, including but not limited to allergic, neurologic, inflammatory, vascular, and autoimmune disorders, are available for participants through the data extraction date of 01 September 2022.
Serious adverse events were reported by 3 participants (3/286, 1.0%) in the monovalent vaccine (Omicron BA.1) group, 2 participants (2/274, 0.7%) in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group, and 2 participants (2/269, 0.7%) in the bivalent vaccine (Original and Omicron BA.1) group. None of these serious adverse events were considered related to vaccination.
Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent), Monovalent Vaccine (Omicron BA.5), Bivalent Vaccine (Original and Omicron BA.5) Administered as a Booster Dose Following Primary and Booster Vaccination with Another Authorized or Approved COVID-19 Vaccine in Individuals 18 Years or Older
The safety of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), the monovalent vaccine (Omicron BA.5), and the bivalent vaccine (Original and Omicron BA.5) administered as a booster dose to individuals 18 years of age and older previously vaccinated with three or more doses of an authorized or approved mRNA COVID-19 vaccine was assessed in a randomized, observer blind study (NCT05372588, Part 2 in Australia; Study 5).
The safety analysis set included 251 participants in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group, 254 participants in the monovalent vaccine (Omicron BA.5) group and 259 participants in the bivalent vaccine (Original and Omicron BA.5) group. The median time since the last COVID-19 vaccination was 352.5 days. The median age of the population was 43.0 years (range 18-83); 632 (82.7%) participants were 18 through 54 years of age and 132 (17.3%) were 55 years and older. Overall, 45.0% were male, 55.0% were female, 2.1% were Hispanic or Latino, 80.5% were White, 0.3% were African American, 2.0% were Aboriginal Australian, 12.3% were Asian, 0.7% were Native Hawaiian or Pacific Islander, 3.1% were other races, and 0.9% were Multiracial. Demographic characteristics were similar across the three groups. Safety analysis included a median follow-up of 70 days post booster dose through data extraction of 22 June 2023. The safety follow-up is ongoing.
Solicited Adverse Reactions
Local and systemic adverse reactions were solicited within 7 days following vaccination with Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), the monovalent vaccine (Omicron BA.5), or the bivalent vaccine (Original and Omicron BA.5) using an electronic diary. The reported frequency and severity of solicited local and systemic adverse reactions are presented for participants 18 years of age and older in Table 7.
Table 7 Number and Percentage of Participants with Solicited Local and Systemic Adverse Reactions Starting within 7* Days After Booster Dose in Participants 18 Years of Age and Older Who Received Primary and Booster Vaccination with Another Authorized or Approved COVID-19 Vaccine (Safety Analysis Set)† Event Monovalent Vaccine (Omicron BA.5)
N = 252Novavax COVID-19 Vaccine (Original monovalent)
N = 251Bivalent Vaccine (Original and Omicron BA.5)
N = 259- *
- 7 days included day of vaccination and the subsequent 6 days. Events were collected in the electronic diary (eDiary). The analysis included a total of 762 participants who received the booster dose who completed their eDiary.
- †
- Absence of rows for Grade 3 or Grade 4 adverse reactions indicates no events were reported.
- ‡
- Grade 3 pain: Defined as any use of narcotic pain reliever or prevents daily activity.
- §
- Grade 3 tenderness: Defined as significant discomfort at rest.
- ¶
- Grade 3 fever: Defined as 39.0 to 40°C (102.1 to 104°F).
- #
- Grade 3 headache: Defined as significant; any use of narcotic pain reliever or prevents daily activity.
- Þ
- Grade 3 fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as significant; prevents daily activity.
- ß
- Grade 3 nausea or vomiting: Defined as prevents daily activity or requires outpatient IV hydration.
Local Adverse Reactions Pain/tenderness Any Grade 153 (60.7) 166 (66.1) 169 (65.3) Grade 3‡,§ 4 (1.6) 2 (0.8) 2 (0.8) Redness (erythema) Any Grade 5 (2.0) 8 (3.2) 6 (2.3) Swelling Any Grade 8 (3.2) 6 (2.4) 6 (2.3) Systemic Adverse Reactions Fever Any Grade 2 (0.8) 2 (0.8) 4 (1.5) Grade 3¶ 0 0 1 (0.4) Headache Any Grade 73 (29.0) 73 (29.1) 74 (28.6) Grade 3# 4 (1.6) 2 (0.8) 3 (1.2) Fatigue/malaise Any Grade 106 (42.1) 103 (41.0) 97 (37.5) Grade 3Þ 3 (1.2) 7 (2.8) 8 (3.1) Muscle pain (myalgia) Any Grade 59 (23.4) 71 (28.3) 67 (25.9) Grade 3Þ 1 (0.4) 2 (0.8) 2 (0.8) Joint pain (arthralgia) Any Grade 18 (7.1) 20 (8.0) 19 (7.3) Grade 3Þ 0 1 (0.4) 1 (0.4) Nausea or vomiting Any Grade 19 (7.5) 18 (7.2) 19 (7.3) Grade 3ß 1 (0.4) 0 0 Unsolicited Adverse Events (non-serious and serious)
Participants were monitored through 36 days after the booster dose for unsolicited adverse events. Additionally, data for serious adverse events and adverse events of interest, including but not limited to allergic, neurologic, inflammatory, vascular, and autoimmune disorders, were collected through the data extraction date of June 22, 2023 (median follow-up post booster of 70 days).
Serious adverse events were reported by 4 participants (4/254, 1.6%) in the monovalent vaccine (Omicron BA.5) group, 1 participant (1/251, 0.4%) in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group, and 2 participants (2/259, 0.8%) in the bivalent vaccine (Original and Omicron BA.5) group. Two participants reported serious adverse events of cranial nerve palsy, including a serious adverse event of fourth nerve cranial palsy with onset of symptoms 7 days post vaccination and a serious adverse event of sixth nerve palsy with onset of symptoms 14 days post vaccination. Both participants had predisposing risk factors, including diabetes, hypertension, hypercholesterolemia. Currently available information on cranial palsies is insufficient to determine a causal relationship with the vaccine. The remaining serious adverse events were not related to vaccination.
Additionally, the safety of a Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) booster dose in individuals who completed a primary vaccination with another authorized or approved COVID-19 vaccine (heterologous booster dose) is inferred from the report of an independent, multicenter, randomized, controlled, Phase 2, trial conducted in the United Kingdom (ISRCTN 73765130). This study was conducted in adults aged 30 years and older with no history of laboratory-confirmed SARS-CoV-2 infection. One study group (n=114 participants; median age 63 years) received Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) administered at least 84 days (median 105 days) after completion of the Pfizer-BioNTech COVID-19 Vaccine primary series. Reported adverse reactions through 28 days following a Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) booster dose did not identify any new safety concerns, as compared with adverse reactions reported following two doses of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) given as a primary series.
Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent), Administered as a Booster Dose Following a Primary Series of Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) in Adolescents 12 Through 17 Years of Age
In an open label portion of Study 1, participants 12 years through 17 years of age (N=1,499) received a single booster dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) at least 5 months after the two-dose primary series (median of 10 months between completion of primary series and booster dose). Safety analyses included evaluation of solicited local and systemic adverse reactions within 7 days after a booster dose, nonserious unsolicited adverse events within 28 days after a booster dose, and serious adverse events for the duration of participation, with data available through a median follow-up of 6.6 months post booster dose through data extraction of November 12, 2022 (94.0% of participants had completed 6 months of safety follow-up).
Among the 1,499 participants, 53.8% were male, 46.2% were female; 73.1% were White, 14.6% were Black or African American, 3.5% were Asian, 2.7% were American Indian (including Native Americans) or Alaskan Native, 0.3% were Native Hawaiian or Other Pacific Islander, and 5.1% were multiple races; 18.4% were Hispanic or Latino.
Solicited Adverse Reactions
Local and systemic adverse reactions were solicited within 7 days following the booster dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) using an electronic diary. The reported frequency and severity of solicited local and systemic adverse reactions for a randomly selected subset of 190 participants 12 through 17 years of age who completed their eDiary is presented in Table 8.
Table 8 Number and Percentage of Participants with Solicited Local and Systemic Adverse Reactions Starting Within 7 Days* After Booster Dose in Participants 12 Years through 17 Years of Age (Booster Safety Analysis Set†)‡ Event Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) N = 190
n (%)- *
- 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (eDiary).
- †
- The analysis included a total of 190 participants who received the booster dose who completed their eDiary
- ‡
- Absence of rows for Grade 4 adverse reactions indicates no events were reported.
- §
- Grade 3 pain: Defined as any use of narcotic pain reliever or prevents daily activity.
- ¶
- Grade 3 tenderness: Defined as significant discomfort at rest.
- #
- Grade 3 redness (erythema): Defined as > 10 cm.
- Þ
- Grade 3 swelling: Defined as > 10 cm or prevents daily activity.
- ß
- Grade 3 fever: Defined as 39.0 to 40°C (102.1 to 104°F).
- à
- Grade 3 headache: Defined as significant; any use of narcotic pain reliever or prevents daily activity.
- è
- Grade 3 fatigue/malaise, muscle pain (myalgia), joint pain (arthralgia): Defined as significant; prevents daily activity.
- ð
- Grade 3 nausea or vomiting: Defined as prevents daily activity or requires outpatient IV hydration.
Local Adverse Reactions Pain/tenderness Any Grade 153 (80.5) Grade 3§,¶ 20 (10.5) Redness (erythema) Any Grade 20 (10.5) Grade 3# 4 (2.1) Swelling Any Grade 19 (10.0) Grade 3Þ 2 (1.1) Systemic Adverse Reactions Fever Any Grade 44 (23.2) Grade 3ß 12 (6.3) Headache Any Grade 130 (68.4) Grade 3à 25 (13.2) Fatigue/malaise Any Grade 132 (69.5) Grade 3è 55 (28.9) Muscle pain (myalgia) Any Grade 117 (61.6) Grade 3è 26 (13.7) Joint pain (arthralgia) Any Grade 43 (22.6) Grade 3è 9 (4.7) Nausea or vomiting Any Grade 50 (26.3) Grade 3ð 5 (2.6) Unsolicited Adverse Events (non-serious and serious)
In Study 1, participants were monitored for non-serious unsolicited adverse events from the first dose through 28 days after the booster dose and for serious adverse events for the duration of study participation.
In a randomly selected subset of 220 participants 12 through 17 years of age, the overall frequency of non-serious unsolicited adverse events through 28 days following the booster dose was 5.0%, including 2 events of lymphadenopathy.
In this open label portion of Study 1, no related serious adverse events were reported in participants 12 years through 17 years of age (N=1,499) through a median safety follow-up of 6.6 months.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-authorization use of the Novavax COVID-19 Vaccine, Adjuvanted. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac Disorders: myocarditis, pericarditis
Immune System Disorders: anaphylaxis
Nervous System Disorders: paresthesia, hypoesthesia
6.3 Required Reporting for Adverse Events and Vaccine Administration Errors
Vaccination providers must report the listed events following administration of the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) to the Vaccine Adverse Event Reporting System (VAERS):
- Vaccine administration errors whether or not associated with an adverse event
- Serious adverse events* (irrespective of attribution to vaccination)
- Cases of myocarditis
- Cases of pericarditis
- Cases of Multisystem Inflammatory Syndrome (MIS)
- Cases of COVID-19 that result in hospitalization or death
*Serious adverse events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
Instructions for Reporting to VAERS
Vaccination providers should complete and submit a VAERS form to FDA using one of the following methods:
- Complete and submit the report online: https://vaers.hhs.gov/reportevent.html, or
- If you are unable to submit this form electronically, you may fax it to VAERS at 1-877-721-0366. If you need additional help submitting a report, you may call the VAERS toll-free information line at 1-800-822-7967 or send an email to info@vaers.org.
IMPORTANT: When reporting adverse events or vaccine administration errors to VAERS, please complete the entire form with detailed information. It is important that the information reported to the FDA be as detailed and as complete as possible. Information to include:
- Patient demographics (e.g., patient name, date of birth)
- Pertinent medical history
- Pertinent details regarding admission and course of illness
- Concomitant medications
- Timing of adverse event(s) in relationship to administration of the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula)
- Pertinent laboratory and virology information
- Outcome of the event and any additional follow-up information if it is available at the time of the VAERS report. Subsequent reporting of follow-up information should be completed if additional details become available.
The following steps are highlighted to provide the necessary information for safety tracking:
- In Box 17, provide information on the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) and any other vaccines administered on the same day; and in Box 22, provide information on any other vaccines received within one month prior.
- In Box 18, description of the event:
- Write “Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) EUA” as the first line.
- Provide a detailed report of vaccine administration error and/or adverse event. It is important to provide detailed information regarding the patient and adverse event/medication error for ongoing safety evaluation of this unapproved vaccine. Please see information to include listed above.
- Contact information:
- In Box 13, provide the name and contact information of the prescribing healthcare provider or institutional designee who is responsible for the report.
- In Box 14, provide the name and contact information of the best doctor/healthcare professional to contact about the adverse event.
- In Box 15, provide the address of the facility where vaccine was given (NOT the healthcare provider’s office address).
Other Reporting Instructions
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to Novavax, Inc. using the contact information below or by providing a copy of the VAERS form to Novavax, Inc.
Website
Fax number
Telephone number
1-888-988-8809
1-844-NOVAVAX
(1-844-668-2829) - 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the Novavax COVID-19 Vaccine, Adjuvanted during pregnancy. Women who are vaccinated with the Novavax COVID-19 Vaccine, Adjuvanted during pregnancy are encouraged to enroll in the registry by visiting https://c-viper.pregistry.com/.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Available data on the Novavax COVID-19 Vaccine, Adjuvanted administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study was performed in female rats administered a vaccine formulation containing the same quantity of SARS-CoV-2 recombinant spike (rS) protein and one-fifth the quantity of adjuvant and formulation buffer inactive ingredients included in Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) on four occasions, twice prior to mating and twice during gestation. This study revealed no evidence of harm to the fetus due to the vaccine. (see Animal Data)
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant individuals infected with SARS-CoV-2 are at increased risk of severe COVID-19 compared with non-pregnant individuals.
Data
In a developmental toxicity study, 0.1 mL of a vaccine formulation containing the same quantity of SARS-CoV-2 rS protein (5 mcg), one-fifth the quantity of adjuvant (10 mcg), and inactive ingredients which comprise the formulation buffer (25 mM sodium phosphate, 300 mM sodium chloride, and 0.01% (w/v) polysorbate 80) contained in a single dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) was administered to female rats by the intramuscular route on four occasions: 27 and 13 days prior to mating, and on gestational days 7 and 15. No vaccine-related adverse effects on female fertility, fetal development, or postnatal development were reported in the study.
8.2 Lactation
Risk Summary
It is not known whether Novavax COVID-19 Vaccine, Adjuvanted is excreted in human milk. Data are not available to assess the effects of the Novavax COVID-19 Vaccine, Adjuvanted on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Novavax COVID-19 Vaccine, Adjuvanted and any potential adverse effects on the breastfed child from Novavax COVID-19 Vaccine, Adjuvanted from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Novavax COVID-19 Vaccine, Adjuvanted is authorized for use in individuals 12 through 17 years of age.
Novavax COVID-19 Vaccine, Adjuvanted is not authorized for use in individuals younger than 12 years of age.
8.5 Geriatric Use
Clinical studies that evaluated primary vaccination with the Novavax COVID-19 Vaccine, Adjuvanted included participants 65 years of age and older receiving vaccine or placebo, and their data contribute to the overall assessment of safety and efficacy. In an ongoing Phase 3 clinical study (Study 1), 12.6% (n=2,480 Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), n=1,235 placebo) of participants were 65 years of age and older and 1.8% (n=361 Novavax COVID-19 Vaccine, Adjuvanted ([Original monovalent)], n=179 placebo) of participants were 75 years of age and older. Vaccine efficacy in participants 65 years of age and older was 78.6% (95% CI: -16.6%, 96.1%) relative to 90.7% (95% CI: 72.9%, 96.8%) in participants 50 through 64 years of age. [see Clinical Trial Results and Supporting Data for EUA (14)]. Overall, there were no notable differences in the safety profiles observed between participants 65 years of age and older and younger participants. [see Adverse Reactions (6.1)]
In a clinical study (Study 1) that evaluated a booster dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), 15.7% (n=2006) of participants were 65 years of age and older and 2.6% (n=326) of participants were 75 years of age and older. Overall, there were no notable differences in the safety profiles observed between participants 65 years of age and older and younger participants. [see Adverse Reactions (6.1)]
-
11 DESCRIPTION
The Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) is a colorless-to-slightly yellow, clear-to-mildly opalescent suspension for intramuscular injection that is free from visible particles. Each 0.5 mL dose of the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) contains 5 mcg of recombinant spike (rS) protein from the SARS-CoV-2 Omicron variant lineage XBB.1.5 and 50 mcg Matrix-M adjuvant. The Matrix-M adjuvant is composed of Fraction-A (42.5 mcg) and Fraction-C (7.5 mcg) of saponin extracts from the soapbark tree, Quillaja saponaria Molina.
The rS protein is produced by recombinant DNA technology using a baculovirus expression system in an insect cell line that is derived from Sf9 cells of the Spodoptera frugiperda species.
Each 0.5 mL dose of the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) also contains the following ingredients: cholesterol, phosphatidylcholine, potassium dihydrogen phosphate (3.85 mcg), potassium chloride (2.25 mcg), disodium hydrogen phosphate dihydrate (14.7 mcg), disodium hydrogen phosphate heptahydrate (2.465 mg), sodium dihydrogen phosphate monohydrate (0.445 mg), sodium chloride (8.766 mg) and polysorbate 80 (0.050 mg). The pH is adjusted with sodium hydroxide or hydrochloric acid.
Each 0.5 mL dose of the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) may also contain residual amounts of baculovirus and Sf9 cell proteins (≤ 0.96 mcg), baculovirus and cellular DNA (≤ 0.00016 mcg), lentil lectin (< 0.025 mcg), methyl-α-D-mannopyranoside (2 mcg), simethicone (< 0.92 mcg), pluronic F-68 (< 2.19 mcg), Triton X-100 (< 0.025 mcg), and Tergitol (NP9) (< 0.05 mcg).
The Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) does not contain a preservative.
The vial stoppers are not made with natural rubber latex.
- 12 CLINICAL PHARMACOLOGY
-
14 CLINICAL TRIAL RESULTS AND SUPPORTING DATA FOR EUA
The effectiveness of Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) is based on effectiveness of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and the immunogenicity of the monovalent vaccine (Omicron BA.1) and monovalent vaccine (Omicron BA.5).
14.1 Efficacy of Two-Dose Primary Series of the Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) in Participants 18 Years of Age and Older
Study 1 is an ongoing Phase 3, multicenter, randomized, observer-blinded, placebo-controlled study in participants 18 years of age and older in United States and Mexico.
Upon enrollment, participants were stratified by age (18 through 64 years or 65 years of age and older). The study excluded participants who were significantly immunocompromised due to immunodeficiency disease; had active cancer on chemotherapy; received chronic immunosuppressive therapy or received immunoglobulin or blood-derived products within 90 days; were pregnant or breastfeeding; or had a history of laboratory-confirmed diagnosed COVID-19. Participants with clinically stable underlying comorbidities were included as were participants with well-controlled human immunodeficiency virus (HIV) infection.
A total of 29,945 participants were randomized in a 2:1 ratio to receive two doses of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) or placebo 3 weeks apart. Assessments of safety and efficacy against COVID-19 are planned for up to 24 months after the second dose.
The primary efficacy analysis population (Per Protocol Efficacy [PP-EFF] Analysis Set) included 25,657 participants who received either the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) (n=17,272) or placebo (n=8,385), received two doses (Dose 1 on day 0; Dose 2 on day 21 median 21 days, range 14-60), did not experience an exclusionary protocol deviation, and did not have evidence of SARS-CoV-2 infection through 6 days after the second dose. In the PP-EFF Analysis Set, 48.5% were female; 21.5% were Hispanic or Latino; 75.9% were White, 11.0% were Black or African American, 6.2% were American Indian or Alaska Native, 4.4% were Asian, and 1.7% were multiracial. The median age of participants was 47 years (range 18-95 years) and 11.7% were 65 years of age and older. Of the study participants in the PP-EFF Analysis Set, 95.2% were at high risk for COVID-19 due to living or working conditions involving known frequent exposure to SARS-CoV-2, comorbidities (chronic lung disease, cardiovascular disease, chronic liver disease, severe obesity, and diabetes), or age ≥ 65 years. Between participants who received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and those who received placebo, there were no notable differences in demographics or pre-existing medical conditions. Participants in the PP-EFF Analysis Set were included in the primary efficacy analysis up until the time that they received their crossover vaccination. As of the September 27, 2021, data cutoff date, the PP-EFF Analysis Set had a median follow-up of 2.5 months post-Dose 2 during the pre-crossover period.
Efficacy of a Primary Series in Participants 18 Years of Age and Older
Vaccine efficacy in participants without evidence of SARS-CoV-2 infection through 6 days after the second dose is presented in Table 9. Based on data accrued through September 27, 2021, the efficacy of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) to prevent polymerase chain reaction (PCR)-confirmed symptomatic mild, moderate or severe COVID-19 from 7 days after Dose 2 was 90.4% (95% CI: 83.8%, 94.3%). In the PP-EFF Analysis Set, no cases of moderate or severe COVID-19 were reported in participants who had received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), compared with nine cases of moderate COVID-19 and four cases of severe COVID-19 reported in participants who had received placebo.
Table 9 Vaccine Efficacy Against PCR-confirmed COVID-19 with Onset from 7 Days After Second Vaccination * (PP-EFF Analysis Set) Subgroup Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) Placebo Vaccine Efficacy (95% CI) (%) Partici-pants
NCOVID-19 Cases
n (%)Mean Incidence Rate Per 1,000 Person-Years† Partici-pants
NCOVID-19 Cases
n (%)Mean Incidence Rate Per 1,000 Person-Years† - *
- Vaccine efficacy (VE) evaluated in participants without major protocol deviations who are seronegative (for SARS-CoV-2) at baseline and do not have a laboratory confirmed current SARS-CoV-2 infection with symptom onset through 6 days after the second dose, and who have received two doses of vaccine or placebo as randomized.
- †
- Mean incidence rate per 1,000 person-years was estimated with weighting for age strata reflective of the distribution seen in the study population.
- ‡
- Based on log-linear model of PCR-confirmed COVID-19 infection incidence rate using Poisson regression with treatment group and age strata as fixed effects and robust error variance, where VE = 100 × (1 – ratio of incidence rate) (Zou 2004).
- §
- Met primary efficacy endpoint criterion for success with a lower bound confidence interval (LBCI) > 30% at the planned primary confirmatory analysis.
Primary efficacy endpoint All participants 17,272 17 (0.1) 5.59 8,385 79 (0.9) 58.30 90.4
(83.8, 94.3) ‡,§Mild - 17 (0.1) - - 66 (0.8) - - Moderate - 0 - - 9 (0.1) - - Severe - 0 - - 4 (< 0.1) - - Descriptive analyses of efficacy showed efficacy point estimates similar to the estimate for the overall study population across genders and racial groups, and across participants with or without medical comorbidities associated with high risk of severe COVID-19. Vaccine efficacy in participants of Hispanic/Latino ethnicity was 77.0% (95% CI: 48.7%, 89.7%) relative to 94.2% (95% CI: 87.9%, 97.2%) in participants who were not Hispanic/Latino. Vaccine efficacy in participants 65 years of age and older was 78.6% (95% CI: -16.6%, 96.1%) relative to 90.7% (95% CI: 72.9%, 96.8%) in participants 50 through 64 years of age.
14.2 Effectiveness of a Two-Dose Primary Series of the Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) in Adolescents 12 Through 17 Years of Age
Effectiveness in adolescents 12 years through 17 years of age is based on a comparison of immune responses in this age group to adults 18 years through 25 years of age.
Study 1 is an ongoing Phase 3 multicenter, randomized, observer-blinded, placebo-controlled study that included 2,247 participants 12 through 17 years of age in the United States. Participants were randomized in a 2:1 ratio to receive two doses of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) or placebo 3 weeks apart. The study excluded participants who were significantly immunocompromised due to immunodeficiency disease; had active cancer on chemotherapy; had received chronic immunosuppressive therapy or had received immunoglobulin or blood-derived products within 90 days; were pregnant or breastfeeding; or had a history of laboratory-confirmed diagnosed COVID-19. Participants with clinically stable underlying comorbidities and participants with well-controlled HIV infection were included.
In Study 1, an analysis was conducted of SARS-CoV-2 neutralizing antibody titers 14 days after Dose 2 in a subset of adolescents 12 through 17 years of age and participants 18 through 25 years of age from the adult main study. Noninferior immune responses as assessed by geometric mean titers and seroconversion rates were demonstrated in a comparison of adolescents 12 through 17 years of age to participants 18 through 25 years of age (Table 10).
Table 10 SARS-CoV-2 Neutralizing Antibody Geometric Mean Titer Ratio and Seroconversion Rate – Comparison of Adolescents 12 Years through 17 Years of Age to Participants 18 Years Through 25 Years of Age – Per-Protocol Immunogenicity Analysis Set Assays Time
Point12 Years Through 17 Years 18 Years Through 25 Years 12 Years Through 17 Years/
18 Years Through 25 YearsGMT*
(95% CI)
n=390GMT*
(95% CI)
n=415GMR†
(95% CI)Met Noninferiority
Criteria‡CI = Confidence interval; GMR = Geometric mean ratio; GMT = Geometric mean titer; SCR = Seroconversion rate - *
- The 95% CI for GMT is calculated based on the t-distribution of the log-transformed values, then back transformed to the original scale for presentation.
- †
- GMR is defined as the ratio of two geometric mean titers for comparison of two age cohorts. An analysis of covariance (ANCOVA) with age cohort as main effect and baseline microneutralization assay neutralizing antibodies as covariate was performed to estimate the GMR.
- ‡
- Noninferiority was achieved if the following 3 pre-specified criteria were met simultaneously: 1) Lower bound of two-sided 95% CI for the ratio of GMTs (GMT12-17yo/GMT18-25yo) > 0.67; 2) Point estimate of the ratio of GMTs ≥ 0.82; and 3) Lower bound of the two-sided 95% CI for difference of SCRs (SCR12-17yo - SCR18-25yo) was > -10%.
- §
- Validated virus neutralizing assay (VNA) with wild-type virus (SARS-CoV-2 hCoV-19/Australia/VIC01/2020 [GenBank MT007544.1]; 360biolabs, Melbourne, Australia). The lower limit for quantification for this assay was a titer of 20, with titers below this level documented as 10.
- ¶
- SCR is defined as percentage of participants with a ≥ 4-fold difference in titers between Day 35 and Day 0. The 95% CI for SCR was calculated using the Clopper-Pearson exact method.
- #
- Difference in SCR in the adolescent primary series expansion (Study 1) for 12 years through 17 years of Study 1 minus SCR in Adult Main Study (Study 1) for 18 years through 25 years. The 95% CI for the difference of SCR between groups was calculated with the method of Miettinen and Nurminen.
SARS-CoV-2 wild-type
microneutralization
assay (1/dilution)§14 days
after
Dose 23859.6
(3422.8, 4352.1)2611.8
(2367.4, 2881.5)1.47
(1.26, 1.72)3Yes SCR%¶
(95% CI)
n=385SCR%¶
(95% CI)
n=414Difference in
SCR%#
(95% CI)98.7
(97.0, 99.6)99.8
(98.7, 100.0)-1.04
(-2.75, 0.20)A descriptive efficacy analysis evaluating PCR-confirmed symptomatic mild, moderate or severe COVID-19 cases was performed in 1,799 participants who were included in the per-protocol efficacy (PP-EFF) Analysis Set, which required receipt of two doses (Dose 1 on day 0; Dose 2 on day 21), no exclusionary protocol deviation(s), and no evidence of SARS-CoV-2 infection through 6 days after the second dose. In the PP-EFF Analysis Set, 47.2% were female; 15.8% were Hispanic or Latino; 76.1% were White, 12.9% were Black or African American, 1.1% were American Indian or Alaska Native, 3.6% were Asian, and 5.6% were multiracial. The median age of participants was 14 years (range 12-17 years). Of the study participants in the PP-EFF Analysis Set, 25.3% were obese. Between participants who received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and those who received placebo, there were no notable differences in demographics. The median interval between doses of study vaccine was 22 days (range 14-43). As of the August 9, 2021, data cutoff date, the PP-EFF Analysis Set had a median follow-up of 67 days post-Dose 2 during the pre-crossover period.
Vaccine efficacy in participants without evidence of SARS-CoV-2 infection through 6 days after the second dose is presented in Table 11. Based on data accrued through August 9, 2021, the efficacy of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) to prevent PCR-confirmed symptomatic mild, moderate or severe COVID-19 from 7 days after Dose 2 was 78.29% (95% CI: 37.55%, 92.45%). No cases of moderate or severe COVID-19 were reported in participants who had received the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) or placebo.
Table 11 Vaccine Efficacy Against PCR-confirmed COVID-19 with Onset from 7 Days After Second Vaccination* (PP-EFF Analysis Set) Subgroup Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) Placebo Vaccine Efficacy (95% CI) (%) Partici-pants
NCOVID-19
Cases†
n (%)Mean Incidence Rate Per 100 Person-Years Partici-pants
NCOVID-19
Cases†
n (%)Mean Incidence Rate Per 100 Person-Years - *
- Vaccine efficacy (VE) evaluated in participants without major protocol deviations who were seronegative (for SARS-CoV-2) at baseline and did not have a laboratory confirmed current SARS-CoV-2 infection with symptom onset through 6 days after the second dose, and who had received two doses of vaccine or placebo as randomized.
- †
- All cases for which sequence data are available (vaccine n=2; placebo n=7) were due to the Delta variant.
- ‡
- Based on Modified Poisson regression with logarithmic link function and treatment group as fixed effect and robust error variance (Zou 2004).
Primary efficacy endpoint All participants 1205 5 (0.4) 2.69 594 11 (1.9) 12.38 78.29
(37.55, 92.45)‡Mild - 5 (0.4) - - 11 (1.9) - - Moderate - 0 - - 0 - - Severe - 0 - - 0 - - 14.3 Immunogenicity of a Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) Booster Dose Following a Novavax COVID-19 Vaccine, Adjuvanted Primary Series in Participants 18 Years and Older
Effectiveness of a booster dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) was based on assessment of neutralizing antibody titers (MN50) against the original SARS-CoV-2 strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020). Immunogenicity analyses compared the MN50 titers following the booster dose to the MN50 titers following the primary series.
In the open-label booster phase of Study 1, participants 18 years of age and older received a single booster dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) at least 6 months after completion of the primary series. A subset of 243 participants were included in the per-protocol immunogenicity (PP-IMM) analysis set, did not have serologic or virologic evidence (if available) of SARS-CoV-2 infection up to 28 days post booster dose. Among participants assessed for immunogenicity, 87.2% were 18-64 years of age, 12.8 % were 65 years of age and older, 51.0% were males, 49.0% were female; 15.6% were Hispanic or Latino; 81.5% were White, 11.1% were Black or African American, 0.4% were American Indian or Alaska Native, 4.9% were Asian, and 1.6% were multiracial. The median age of participants was 52 years (range 19-79 years).
Prespecified immunogenicity non-inferiority analyses included an assessment of MN50 geometric mean titer (GMT) ratio and difference in seroconversion rates. Seroconversion for a participant was defined as achieving a 4-fold rise in MN50 from baseline (before the booster dose and before the first dose of the primary series).
The analysis of the GMT ratio of MN50 following the booster dose compared with the primary series met the non-inferiority criteria for a booster response (lower limit of the 95% CI > 0.67 and point estimate > 0.83).
The lower limit of the two-sided 95% CI for the difference in seroconversion rates (percentage) was -14.4%, which did not meet the non-inferiority criteria for a booster response (lower limit of 95% CI for the percentage difference of ≥ -10%). These analyses are summarized in Table 12 and Table 13.
Table 12 Neutralizing Antibody Geometric Titers (MN50) Against the Original SARS-CoV-2 Virus Strain (SARS CoV-2 hCoV-19/Australia/VIC01/2020) at 28 Days after a Booster Dose Versus 14 Days After Completion of the Primary Series, Participants 18 Years of Age and Older, PP-IMM Analysis Set* Booster Dose
(N = 239)†
GMT
(95% CI)‡Primary Series
(N = 239)
GMT
(95% CI) ‡GMT Ratio
(Booster/Primary Series)
(95% CI)*Met Success Criteria Abbreviations: CI = confidence interval; GMT = geometric mean titer; MN50 = microneutralization assay with an inhibitory concentration of 50%; PP-IMM = Per-Protocol Immunogenicity. Note: The median duration between the time of the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and the time of the booster dose was 10 months. - *
- PP-IMM Analysis Set included participants who received two doses (0.5 mL 3 weeks apart) of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the initial vaccination period or in the blinded crossover vaccination period, had an immunogenicity blood sample collected at Day 35, did not have serologic or virologic evidence (if available) of SARS-CoV-2 infection up to 28 days post booster dose, did not receive an emergency use authorized COVID-19 vaccine, received the booster dose, and remained blinded on study and without major protocol deviations until 7 days post-crossover Dose.
- †
- The analysis included a total of 239 participants of the PP-IMM analysis set who had immunogenicity data available for both the booster and primary series.
- ‡
- The 95% CI for GMT and GMT ratio were calculated based on the t-distribution of the log-transformed values, then back transformed to the original scale for presentation.
5075.6
(4448.3, 5791.4)1505.7
(1244.1, 1822.3)3.4
(2.8, 4.0)Lower limit of 95% CI > 0.67 and point estimate > 0.83 criteria: Yes Table 13 Seroconversion Rates (%) Against the Original SARS-CoV-2 Strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) at 28 Days after a Booster Dose Versus 14 Days After Completion of the Primary Series, Participants 18 Years of Age and Older, PP-IMM Analysis Set* Booster Dose
(N = 239)†
SCR
% (n)
(95% CI)‡Primary Series
(N = 239)
SCR
%(n)
(95% CI) ‡Difference in SCR§
(Booster-Primary Series)
(95% CI)¶Met Success Criteria# Abbreviations: CI = confidence interval; PP-IMM = Per-Protocol Immunogenicity; SCR = seroconversion rate. Note: SCR was defined as the proportion of participants with post-vaccination levels ≥ 4-fold higher than the baseline levels.
Note: The median duration between the time of the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and the time of the booster dose was 10 months.- *
- PP-IMM Analysis Set included participants who received two doses (0.5 mL 3 weeks apart) of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the initial vaccination period or in the blinded crossover vaccination period, had an immunogenicity blood sample collected at Day 35, did not have serologic or virologic evidence (if available) of SARS-CoV-2 infection up to 28 days post booster dose, did not receive an emergency use authorized COVID-19 vaccine, received the booster dose, and remained blinded on study and without major protocol deviations until 7 days post-crossover Dose 2.
- †
- The analysis included a total of 239 participants of the PP-IMM analysis set who had immunogenicity data (microneutralization) available for both the booster and primary series
- ‡
- 95% CI is based on the Clopper-Pearson method.
- §
- Based on the Tango method.
- ¶
- Comparison between SCR of 28 days post-booster relative to time of booster and SCR of 14 days after second dose of the primary series relative to time of first dose.
- #
- Non-inferiority of the single booster dose was achieved if the lower limit of the 95% CI for the difference of the proportion of participants with SCR at 28 days after a single booster dose relative to the time of booster vaccination versus at 14 days after the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) relative to the time of first vaccination was > -10%.
85.4 (204)
(80.2, 89.6)94.6 (226)
(90.9, 97.1)-9.2%
(-14.4%, -4.5
%)Lower limit of 95% CI > -10% criterion: No An additional descriptive analysis evaluated seroconversion rates using baseline neutralizing antibody titers prior to Dose 1 of the primary series. As shown in Table 14, the booster dose seroconversion rate, with seroconversion defined as at least a 4-fold rise relative to the time of first dose, was 98.3%. The difference in seroconversion rates in this post-hoc analysis was 3.8% (95% CI: 2.0%, 7.0%).
Table 14 Seroconversion Rates (%) Against the Original SARS-CoV-2 Strain (SARS CoV-2 hCoV-19/Australia/VIC01/2020) at 28 Days after a Booster Dose Versus 14 Days After Completion of the Primary Series, Participants 18 Years of Age and Older, PP-IMM Analysis Set* Booster Dose
(N = 239)†
SCR
n % (n)
(95% CI)‡Primary Series
(N = 239)
SCR
n % (n)
(95% CI)‡Difference in SCR§
(Booster-Primary Series)
(95% CI)¶Abbreviations: CI = confidence interval; PP-IMM = Per-Protocol Immunogenicity; SCR = seroconversion rate. Note: SCR was defined as the proportion of participants with post-vaccination levels ≥ 4-fold higher than at the time of the first dose. Note: The median duration between the time of the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and the time of the booster dose was 10 months. - *
- PP-IMM Analysis Set included all participants who received two doses (0.5 mL 3 weeks apart) of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the initial vaccination period or in the blinded crossover vaccination period, had an immunogenicity blood sample collected at Day 35, did not have serologic or virologic evidence (if available) of SARS-CoV-2 infection up to 28 days post booster dose, did not receive an emergency use authorized COVID-19 vaccine, received the booster dose, and remained blinded on study and without major protocol deviations until 7 days post-crossover Dose 2.
- †
- The analysis included a total of 239 participants of the PP-IMM analysis set who had immunogenicity data (microneutralization) available for both the booster and primary series.
- ‡
- 95% CI is based on the Clopper-Pearson method.
- §
- Based on the Tango method.
- ¶
- Comparison between SCR of 28 days post-booster relative to time of first dose and SCR of 14 days after second dose of the primary series relative to time of first dose.
98.3 (235)
(95.8, 99.5)94.6 (226)
(90.9, 97.1)3.8%
(2.0%, 7.0%)14.4 Immunogenicity of a Booster Dose of Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent), Following a Primary Series with Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent), in Participants 12 through 17 Years of Age
Effectiveness of a booster dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) was based on assessment of neutralizing antibody titers (MN50) against the original SARS-CoV-2 strain (SARSCoV-2 hCoV-19/Australia/VIC01/2020). Immunogenicity analyses compared the MN50 titers following the booster dose to the MN50 titers following the primary series in participants who had data at both time points.
In the open-label booster phase of Study 1, participants 12 through 17 years of age received a single booster dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) at least 5 months after completion of the primary series. A subset of 58 participants were included in the per-protocol immunogenicity (PP-IMM) analysis set, had immunogenicity blood samples collected at 14 days after the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and at 28 days after the booster dose, and did not have serologic or virologic evidence of SARS-CoV-2 infection on or before the booster dose. Among participants assessed for immunogenicity, 51.7% were males, 48.3% were female; 17.2% were Hispanic or Latino; 91.4% were White, 1.7% were Black or African American, 1.7% were Asian, and 5.2% were multiracial. The median age of participants was 14 years (range 12-17 years).
Prespecified immunogenicity non-inferiority analyses included an assessment of MN50 GMT ratio and percentage difference in seroconversion rates. Seroconversion for a participant was defined as achieving a 4-fold rise in MN50 from baseline (before the first dose of the primary series).
The analysis of the GMT ratio of MN50 following the booster dose compared with the primary series met the non-inferiority criteria for a booster response (lower limit of the 95% CI > 0.67 and point estimate > 0.83).
The lower limit of the two-sided 95% CI for the difference in seroconversion rates (percentage) was -6.8%, which did meet the non-inferiority criteria for a booster response (lower limit of 95% CI for the percentage difference of ≥ -10%). These analyses are summarized in Table 15 and Table 16.
Table 15 Neutralizing Antibody Geometric Titers (MN50) Against the Original SARS-CoV-2 Virus Strain (SARS CoV-2 hCoV-19/Australia/VIC01/2020) at 28 Days after a Booster Dose Versus 14 Days After Completion of the Primary Series, Participants 12 Years through 17 Years of Age, PP-IMM Analysis Set* Booster Dose
(N = 53)†
GMT
(95% CI)‡Primary Series
(N = 53)
GMT
(95% CI) ‡GMT Ratio
(Booster/Primary Series)
(95% CI)*Met Success Criteria Abbreviations: CI = confidence interval; GMT = geometric mean titer; MN50 = microneutralization assay with an inhibitory concentration of 50%; PP-IMM = Per-Protocol Immunogenicity. Note: The median duration between the time of the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and the time of the booster dose was 10.6 months. - *
- PP-IMM Analysis Set included participants who received two doses (0.5 mL 3 weeks apart) of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the initial vaccination period or in the blinded crossover vaccination period, had an immunogenicity blood sample collected at Day 35 (primary series) and at 28 days after booster vaccination, did not have serologic or virologic evidence of SARS-CoV-2 infection on or before the booster dose, did not receive an emergency use authorized COVID-19 vaccine, received the booster dose, and remained blinded on study and without major protocol deviations until 7 days post-crossover Dose.
- †
- The analysis included a total of 53 participants of the PP-IMM analysis set who had immunogenicity data available for both the booster and primary series.
- ‡
- The 95% CI for GMT and GMT ratio were calculated based on the t-distribution of the log-transformed values, then back transformed to the original scale for presentation.
11824.4
(8993.1, 15546.9)4434.0
(3658.0, 5374.5)2.7
(2.0, 3.5)Lower limit of 95% CI > 0.67 and point estimate > 0.83 criteria: Yes Table 16 Seroconversion Rates (%) Against the Original SARS-CoV-2 Strain (SARS-CoV-2 hCoV-19/Australia/VIC01/2020) at 28 Days after a Booster Dose Versus 14 Days After Completion of the Primary Series, Participants 12 Years through 17 Years of Age, PP-IMM Analysis Set* Booster Dose
(N = 53)†
SCR
%
(95% CI)‡Primary Series
(N = 53)
SCR
%
(95% CI)‡Difference in SCR§
(Booster-Primary Series)
(95% CI)¶Met Success Criterion# Abbreviations: CI = confidence interval; PP-IMM = Per-Protocol Immunogenicity; SCR = seroconversion rate. Note: SCR was defined as the proportion of participants with post-vaccination levels ≥ 4-fold higher than the baseline levels.
Note: The median duration between the time of the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) and the time of the booster dose was 10.6 months.- *
- PP-IMM Analysis Set included participants who received two doses (0.5 mL 3 weeks apart) of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in the initial vaccination period or in the blinded crossover vaccination period, had an immunogenicity blood sample collected at Day 35 (primary series) and at 28 days after booster vaccination, did not have serologic or virologic evidence of SARS-CoV-2 infection on or before the booster dose, did not receive an emergency use authorized COVID-19 vaccine, received the booster dose, and remained blinded on study and without major protocol deviations until 7 days post-crossover Dose..
- †
- The analysis included a total of 53 participants of the PP-IMM analysis set who had immunogenicity data (microneutralization) available for both the booster and primary series
- ‡
- 95% CI is based on the Clopper-Pearson method.
- §
- Based on the Tango method.
- ¶
- Comparison between SCR of 28 days post-booster relative to time of booster and SCR of 14 days after second dose of the primary series relative to time of first dose.
- #
- Non-inferiority of the single booster dose was achieved if the lower limit of the 95% CI for the difference of the proportion of participants with SCR at 28 days after a single booster dose relative to the time of first vaccination versus at 14 days after the second dose of the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) relative to the time of first vaccination was > -10%.
100
(93.3, 100)100
(93.3, 100)0.0
(-6.8, 6.8)LB of 95% CI > -10% criterion: Yes 14.5 Immunogenicity of a Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) Booster Dose Following Primary Vaccination with Another Authorized or Approved COVID-19 Vaccine
Effectiveness of a Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) booster dose in individuals who completed primary vaccination with another authorized or approved COVID-19 vaccine is inferred from immunogenicity data reported from an independent study conducted in the United Kingdom (ISRCTN 73765130). This multicenter, randomized, controlled Phase 2 trial investigated the immunogenicity of a single booster dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) in participants who had received two doses of the Pfizer-BioNTech COVID-19 Vaccine as a primary vaccination series. Participants included adults aged 30 years and older with no history of laboratory-confirmed SARS-CoV-2 infection. The Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) was administered at least 84 days after completion of a Pfizer-BioNTech COVID-19 Vaccine primary series in 114 participants. Neutralizing antibody titers measured by a microneutralization assay were assessed prior to the booster dose and 28 days post-booster dose. A booster response to the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) was demonstrated.
14.6 Immunogenicity of Monovalent Vaccine (Omicron BA.1) and Monovalent Vaccine (Omicron BA.5) Doses Following Primary and Booster Vaccination with Another Authorized or Approved COVID-19 Vaccine in Participants 18 Years of Age and Older
In Study 5 Part 1, a subgroup of participants 18 to 64 years of age who previously received 3 doses of the Pfizer-BioNTech COVID-19 Vaccine or the Moderna COVID-19 Vaccine, received one of the following as a booster dose: Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) or monovalent vaccine (Omicron BA.1). The booster doses were administered at a median of 182 and 177 days after the last vaccination, respectively. Neutralizing antibody titers for the Omicron BA.1 virus, measured by a microneutralization assay [MN50], were evaluated at 14 days after vaccination. Participants included in the day 14 per protocol analysis set population (n=240) had no serologic or virologic evidence of SARS-CoV-2 infection prior to the booster dose.
Prespecified immunogenicity analyses included an assessment of MN50 GMT ratio and difference in seroresponse rates. Seroresponse rate was defined as the percentage of participants achieving a 4-fold rise in MN50 from baseline (before the first dose of the study vaccine).
The analysis of the GMT ratio following the booster dose with monovalent vaccine (Omicron BA.1) compared to the booster dose with Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) met the superiority criterion for success (lower limit of the 95% CI > 1.0).
The lower limit of the two-sided 95% CI for the difference in seroresponse rates (percentage) was 10.3%, which met the non-inferiority criterion for success (lower limit of 95% CI for the percentage difference of > -5%). These analyses are summarized in Table 17 and Table 18.
Table 17 Summary of Geometric Mean Titers of Monovalent Vaccine (Omicron BA.1) Against the Omicron BA.1 Virus at 14 Days After a Booster Dose Versus the Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) at 14 Days After a Booster Dose, Participants 18 Years through 64 Years of Age, PP Analysis Set* Monovalent vaccine (Omicron BA.1)
(N = 124)†
GMT
(95% CI)‡Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)
(N = 116)†
GMT
(95% CI)‡GMT Ratio§
[Monovalent vaccine (Omicron BA.1)/Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)]
(95% CI)§Met Success Criterion Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; GMT = geometric mean titer; MN50 = microneutralization assay with an inhibitory concentration of 50%; PP = Per-Protocol; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. - *
- PP Analysis Set included participants who received study vaccine according to protocol, did not have serologic or virologic evidence of SARS-CoV-2 infection on or before the booster dose, and had no major protocol violations that were considered clinically relevant to impact immunogenicity.
- †
- The analysis included participants of the PP analysis set who had immunogenicity data available at baseline and at 14 days post booster dose.
- ‡
- The 95% CI for GMT were calculated based on the t-distribution of the log-transformed values, then back transformed to the original scale for presentation.
- §
- An ANCOVA with vaccine group as fixed effect and baseline value as covariate was performed to estimate the GMT ratio. The mean difference between vaccine groups and the corresponding CI limits was then exponentiated to obtain the ratio of MN50 GMTs and the corresponding 95% CIs.
- ¶
- Success criterion is met if the lower bound of the two-sided 95% CI was above unity (ie, > 1).
130.8
(109.2, 156.7)83.9
(69.6, 101.2)1.6
(1.33, 2.03)Yes¶ Table 18 Summary of Seroresponse Rate of Monovalent Vaccine (Omicron BA.1) Against the Omicron BA.1 Virus at 14 Days After a Booster Dose Versus the Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) at 14 Days After a Booster Dose, Participants 18 Years through 64 Years of Age, PP Analysis Set* Monovalent vaccine (Omicron BA.1)
(N = 124)†
SRR‡
%
(95% CI)§Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)
(N = 116)†
SRR‡
%
(95% CI)§Difference in SRR
[(Monovalent vaccine (Omicron BA.1) - Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)]
%
(95% CI)¶Met Success Criterion Abbreviations: CI = confidence interval; MN50 = microneutralization assay with an inhibitory concentration of 50%; PP = Per-Protocol; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SRR = seroresponse rate. - *
- PP Analysis Set included participants who received study vaccine according to protocol, did not have serologic or virologic evidence of SARS-CoV-2 infection on or before the booster dose, and had no major protocol violations that were considered clinically relevant to impact immunogenicity.
- †
- The analysis included participants of the PP analysis set who had immunogenicity data available at baseline and at 14 days post booster dose.
- ‡
- The SRR was defined as percentage of participants at each post vaccination visit with a titer ≥ 4-fold rise in MN50 level.
- §
- The 95% CI for SRR was calculated using the exact Clopper-Pearson method.
- ¶
- The 95% CI for the difference in SRR was calculated based on the method of Miettinen and Nurminen.
- #
- Success criterion is met if the lower bound of the two-sided 95% CI was above -5%.
73.4
(64.7, 80.9)50.9
(41.4, 60.3)22.5
(10.3, 34.2)Yes# In sensitivity analyses using a per protocol analysis set that did not exclude participants with serologic evidence of SARS-CoV-2 infection (PP2 Analysis Subset, n= 491), neutralizing antibody responses against the Omicron BA.1 virus induced by the monovalent vaccine (Omicron BA.1) were compared with neutralizing antibody responses against the Omicron BA.1 virus induced by the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) 14 days after study vaccination.
The GMTs were 318.2 (95% CI: 269.8, 375.3) in the monovalent vaccine (Omicron BA.1) group (n= 247) and 218.1 (95% CI: 186.0, 255.7) in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group (n= 244), resulting in an estimated GMT ratio of the monovalent vaccine (Omicron BA.1) versus the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) of 1.5 (95% CI: 1.36, 1.77).
The seroresponse rates (percentage) were 54.3% in the monovalent vaccine (Omicron BA.1) group and 32.0% in the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) group, resulting in a difference in seroresponse rates (percentage) of 22.3% (95% CIs: 13.6%, 30.6%).
In Study 5 Part 2, a subgroup of participants 18 years of age and older who previously received at least 3 doses of the Pfizer-BioNTech COVID-19 Vaccine or the Moderna COVID-19 Vaccine, received one of the following as a booster dose: Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) or monovalent vaccine (Omicron BA.5). The booster doses were administered a median of 389 and 328 days after the last vaccination, respectively. Neutralizing antibody titers against a pseudovirus expressing the SARS-CoV-2 Spike protein from the Omicron BA.5 virus, measured by pseudovirus neutralization assay [ID50], were evaluated at 28 days after vaccination. Participants included in the day 28 per protocol analysis set population (n=462) had no virologic evidence of SARS-CoV-2 infection at time of the booster dose.
Exploratory immunogenicity analyses included an assessment of the ID50 GMT ratio and difference in seroresponse rates. Seroresponse rate was defined as the percentage of participants achieving a 4-fold rise in ID50 from baseline (before the first dose of the study vaccine).
The GMT ratio following the booster dose with monovalent vaccine (Omicron BA.5) compared with the booster dose with Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) was 2.5 (two-sided 95% confidence interval: 2.10, 2.94).
The difference in seroresponse rates (percentage) between the booster dose with monovalent vaccine (Omicron BA.5) and the booster dose with Novavax Vaccine, Adjuvanted (Original monovalent) was 33.2% (two-sided 95% confidence interval: 25.4%, 40.7%). These analyses are summarized in Table 19 and Table 20.
Table 19 Summary of Geometric Mean Titers of Monovalent Vaccine (Omicron BA.5) Against a Pseudovirus Expressing the SARS-CoV-2 Spike Protein from Omicron BA.5 sublineage at 28 Days After a Booster Dose Versus the Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) at 28 Days After a Booster Dose, Participants 18 Years of Age and Older, PP Analysis Set* Monovalent vaccine (Omicron BA.5)
(N = 235)†
Adjusted GMT
(95% CI)‡Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)
(N = 227)†
Adjusted GMT‡
(95% CI)‡GMT Ratio‡
[Monovalent vaccine (Omicron BA.5)/
Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)]
(95% CI)‡Abbreviations: ANCOVA = analysis of covariance; CI = confidence interval; GMT = geometric mean titer; PP = Per-Protocol; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2. - *
- PP Analysis Set included participants who received study vaccine according to protocol, had serologic or virologic results for baseline and at Day 28, and had no major protocol violations that were considered clinically relevant to impact immunogenicity.
- †
- The analysis included participants of the PP analysis set who had immunogenicity data available at baseline and at 28 days post booster dose.
- ‡
- An ANCOVA with vaccine group and age group (18-54 years, ≥ 55 years) as fixed effects and baseline value (Day 0) as covariate is performed to estimate the adjusted GMT and GMT ratio. The mean difference between vaccine groups and the corresponding CI limits is then exponentiated to obtain the ratio of GMTs and the corresponding 95% CIs.
1279.1
(1119.7, 1461.1)515.1
(450.4, 589.0)2.5
(2.10, 2.94)Table 20 Summary of Seroresponse Rate of Monovalent Vaccine (Omicron BA.5) Against a Pseudovirus Expressing the SARS-CoV-2 Spike Protein from Omicron BA.5 sublineage at 28 Days After a Booster Dose Versus the Novavax COVID-19 Vaccine, Adjuvanted (Original Monovalent) at 28 Days After a Booster Dose, Participants 18 Years of Age and Older, PP Analysis Set* Monovalent vaccine (Omicron BA.5)
(N = 235)†
SRR‡
%
(95% CI)§Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)
(N = 227)†
SRR‡
%
(95% CI)§Difference in SRR
[(Monovalent vaccine (Omicron BA.1) - Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent)]
%
(95% CI)¶Abbreviations: CI = confidence interval; LLOQ = lower limit of quantitation; PP = Per-Protocol; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SRR = seroresponse rate. - *
- PP Analysis Set included participants who received study vaccine according to protocol, had serologic or virologic results for baseline and at Day 28, and had no major protocol violations that were considered clinically relevant to impact immunogenicity.
- †
- The analysis included participants of the PP analysis set who had immunogenicity data available at baseline and at 28 days post booster dose.
- ‡
- The SRR is defined as ≥ 4-fold increase from baseline value if the baseline value is equal to or above LLOQ; or ≥ 4 times the LLOQ if the baseline value is below LLOQ.
- §
- The 95% CI for SRR is calculated using the exact Clopper-Pearson method.
- ¶
- The 95% CI for the difference in SRR was calculated based on the method of Miettinen and Nurminen.
45.5
(39.0, 52.1)12.3
(8.4, 17.3)33.2
(25.4, 40.7) -
16 HOW SUPPLIED/STORAGE AND HANDLING
The Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) is supplied as:
- Carton (NDC 80631-105-02) containing 2 multi-dose vials (NDC 80631-105-01). Each multi-dose vial contains 5 doses of 0.5 mL each.
Storage of Unpunctured Vial
Store the unpunctured multi-dose vaccine vial in a refrigerator between 2 to 8°C (36 to 46°F).
Do not freeze.
Protect from light.
Storage After First Needle Puncture of the Vial
After first puncture, hold the vial between 2 to 25°C (36 to 77°F) for up to 12 hours. Discard the vial 12 hours after the first puncture.
-
17 PATIENT COUNSELING INFORMATION
Advise the recipient or caregiver to read the Fact Sheet for Recipients and Caregivers.
The vaccination provider must include vaccination information in the state/local jurisdiction’s Immunization Information System (IIS) or other designated system. Advise recipient or caregiver that more information about IISs can be found at: https://www.cdc.gov/vaccines/programs/iis/about.html.
-
18 MANUFACTURER INFORMATION
For general questions, visit the website or call the telephone number provided below.
Website
Telephone number
1-844-NOVAVAX
(1-844-668-2829)This Full EUA Prescribing Information may have been updated. For the most recent Full EUA Prescribing Information, please see www.NovavaxCovidVaccine.com.
novavax
Manufactured for:
Novavax, Inc., Gaithersburg, MD, 20878C20001US-XXX
Revised: October 3, 2023
©2023 Novavax, Inc. All rights reserved.
-
INFORMATION FOR PATIENTS
FACT SHEET FOR RECIPIENTS AND CAREGIVERS
EMERGENCY USE AUTHORIZATION (EUA) OF
THE NOVAVAX COVID-19 VACCINE, ADJUVANTED (2023-2024 FORMULA) TO
PREVENT CORONAVIRUS DISEASE 2019 (COVID-19) IN
INDIVIDUALS 12 YEARS OF AGE AND OLDERYou are being offered the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This Fact Sheet contains information to help you understand the risks and benefits of the Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula), hereafter referred to as Novavax COVID-19 Vaccine, Adjuvanted, which you or your child may receive because there is currently a pandemic of COVID-19. Talk to your or your child’s vaccination provider if you have questions.
This Fact Sheet may have been updated. For the most recent Fact Sheet, please see http://www.NovavaxCovidVaccine.com.
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) to make the Novavax COVID-19 Vaccine, Adjuvanted available during the COVID-19 pandemic (for more details about an EUA please see “WHAT IS AN EMERGENCY USE AUTHORIZATION?” at the end of this document). The Novavax COVID-19 Vaccine, Adjuvanted is not an FDA-approved vaccine in the United States. Read this Fact Sheet for information about the Novavax COVID-19 Vaccine, Adjuvanted.
WHAT IS COVID-19?
COVID-19 is caused by a coronavirus called SARS-CoV-2. You can get COVID-19 through contact with another person who has the virus.
It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have had a wide range of symptoms reported, ranging from mild symptoms to severe illness leading to death. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
WHAT IS THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
The Novavax COVID-19 Vaccine, Adjuvanted is a vaccine for use in individuals 12 years of age and older to prevent COVID-19.1 The FDA has authorized the emergency use of the Novavax COVID-19 Vaccine, Adjuvanted under an EUA.
The Novavax COVID-19 Vaccine, Adjuvanted may not protect everyone.
WHAT SHOULD YOU MENTION TO YOUR VACCINATION PROVIDER BEFORE YOU OR YOUR CHILD GETS THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
Tell your vaccination provider about all of your or your child’s medical conditions, including if you or your child:
- •
- have any allergies
- •
- have had myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining outside the heart)
- •
- have a fever
- •
- have a bleeding disorder or are on a blood thinner
- •
- are immunocompromised or are on a medicine that affects your immune system
- •
- are pregnant or plan to become pregnant
- •
- are breastfeeding
- •
- have received another COVID-19 vaccine
- •
- have ever fainted in association with an injection
The Novavax COVID-19 Vaccine, Adjuvanted is given as an injection into the muscle.
Individuals previously vaccinated with one or more doses of a monovalent COVID-19 vaccine2 or a bivalent COVID-19 vaccine 3: A single dose is administered at least 2 months after the last previous dose of any monovalent or bivalent COVID-19 vaccine.
Individuals not previously vaccinated with any COVID-19 vaccine: Two doses are administered 3 weeks apart.
Immunocompromised individuals 12 years of age and older
Additional doses of Novavax COVID-19 Vaccine, Adjuvanted may be administered. For more information, talk to your child’s healthcare provider.
WHO SHOULD NOT GET THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
A person should not get the Novavax COVID-19 Vaccine, Adjuvanted if they had:
- a severe allergic reaction after a previous dose of any Novavax COVID-19 Vaccine, Adjuvanted
- a severe allergic reaction to any ingredient of these vaccines
WHAT ARE THE INGREDIENTS IN THIS VACCINE?
The Novavax COVID-19 Vaccine, Adjuvanted contains a recombinant form of the SARS-CoV-2 spike protein produced from baculovirus infected Sf9 (fall armyworm) insect cells and Matrix-MTM adjuvant containing saponins derived from the soapbark tree (Quillaja saponaria Molina). Other ingredients include cholesterol, phosphatidylcholine, potassium dihydrogen phosphate, potassium chloride, disodium hydrogen phosphate dihydrate, sodium chloride, disodium hydrogen phosphate heptahydrate, sodium dihydrogen phosphate monohydrate, polysorbate 80. The vaccine may also contain small amounts of baculovirus and insect cell proteins and DNA.
HAS THIS VACCINE BEEN USED BEFORE?
Tens of thousands of individuals 12 years of age and older have received Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent) under EUA.
In clinical trials, approximately 28,500 individuals 12 years of age and older have received at least one dose of Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent).
Approximately 1000 individuals have received at least a single dose of a Novavax monovalent or bivalent vaccine containing different spike proteins of SARS-CoV-2.
The Novavax COVID-19 Vaccine, Adjuvanted is made in the same way as the Novavax COVID-19 Vaccine, Adjuvanted (Original monovalent), but it contains the spike protein of SARS-CoV-2 Omicron variant lineage XBB.1.5 (Omicron XBB.1.5).
WHAT ARE THE BENEFITS OF THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
FDA has authorized the Novavax COVID-19 Vaccine, Adjuvanted to provide protection against COVID-19.
The duration of protection against COVID-19 is currently unknown.
WHAT ARE THE RISKS OF THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
There is a remote chance that the vaccine could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose. For this reason, the vaccination provider may ask you or your child to stay at the place where you or your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include:
- •
- Difficulty breathing
- •
- Swelling of the face and throat
- •
- A fast heartbeat
- •
- A bad rash all over the body
- •
- Dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received the vaccine. In most of these people, symptoms began within 10 days following vaccination. The chance of having this occur is very low. You should seek medical attention right away if you or your child have any of the following symptoms after receiving the vaccine:
- •
- Chest pain
- •
- Shortness of breath
- •
- Feelings of having a fast-beating, fluttering, or pounding heart
Side effects that have been reported in clinical trials with the Novavax COVID-19 Vaccine, Adjuvanted include:
- •
- Myocarditis (inflammation of the heart muscle)
- •
- Pericarditis (inflammation of the lining outside the heart)
- •
- Injection site reactions: pain/tenderness, swelling, redness and itching
- •
- General side effects: fatigue or generally feeling unwell, muscle pain, headache, joint pain, nausea, vomiting, fever, chills
- •
- Allergic reactions such as hives and swelling of the face
- •
- Swollen lymph nodes
Side effects that have been reported in post-authorization use with the Novavax COVID-19 Vaccine, Adjuvanted include:
- •
- Severe allergic reactions
- •
- Myocarditis (inflammation of the heart muscle)
- •
- Pericarditis (inflammation of the lining outside the heart)
- •
- Paresthesia (unusual feeling in the skin such as tingling or a crawling feeling), hypoesthesia (decreased feeling or sensitivity, especially in the skin)
These may not be all the possible side effects. Serious and unexpected side effects may occur. The possible side effects are still being studied.
WHAT SHOULD I DO ABOUT SIDE EFFECTS?
If you or your child experience a severe allergic reaction, call 9-1-1, or go to the nearest hospital.
Call the vaccination provider or your healthcare provider for any side effects that bother you or your child or do not go away.
Report vaccine side effects to the FDA and the Centers for Disease Control and Prevention (CDC) Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to https://vaers.hhs.gov/reportevent.html. Please include “Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) EUA” in the first line of box #18 of the report form.
In addition, you can report side effects to Novavax, Inc., at the contact information provided below.
Website
Fax number
Telephone number
1-888-988-8809
1-844-NOVAVAX
(1-844-668-2829)WHAT IF I DECIDE NOT TO GET OR NOT TO HAVE MY CHILD GET THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
Under the EUA, there is an option to accept or refuse receiving this vaccine. If you decide not to receive this vaccine or for your child not to receive this vaccine, it will not change the standard medical care.
ARE THERE OTHER VACCINES FOR PREVENTING COVID-19 BESIDES THE NOVAVAX COVID-19 VACCINE, ADJUVANTED?
Other vaccines for preventing COVID-19 include the FDA-approved COVID-19 vaccines, COMIRNATY (COVID-19 Vaccine, mRNA) and SPIKEVAX (COVID-19 Vaccine, mRNA), for individuals 12 years of age and older.
CAN I OR MY CHILD RECEIVE THE NOVAVAX COVID-19 VACCINE, ADJUVANTED AT THE SAME TIME AS OTHER VACCINES?
Data have not yet been submitted to FDA on administration of Novavax COVID-19 Vaccine, Adjuvanted at the same time as other vaccines. If you are considering having you or your child receive the Novavax COVID-19 Vaccine, Adjuvanted with other vaccines, discuss your options with your healthcare provider.
WHAT IF I AM OR MY CHILD IS IMMUNOCOMPROMISED?
Immunocompromised individuals 12 years of age and older may receive additional doses of Novavax COVID-19 Vaccine, Adjuvanted (see HOW IS THE VACCINE GIVEN? above).
WHAT ABOUT PREGNANCY OR BREASTFEEDING?
If you or your child are pregnant or breastfeeding, discuss the options with your healthcare provider.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the Novavax COVID-19 Vaccine, Adjuvanted during pregnancy. Women who are vaccinated with the Novavax COVID-19 Vaccine, Adjuvanted during pregnancy are encouraged to enroll in the registry by visiting https://c-viper.pregistry.com/.
WILL THIS VACCINE GIVE ME OR MY CHILD COVID-19?
No. This vaccine does not contain SARS-CoV-2 and cannot give you or your child COVID-19.
ADDITIONAL INFORMATION
If you have questions, visit the website or call the telephone number provided below.
To access the most recent Fact Sheets, please scan the QR code provided below.
Novavax COVID-19 Vaccine,
Adjuvanted (2023-2024 Formula)
websiteTelephone number

1-844-NOVAVAX
(1-844-668-2829)
HOW CAN I LEARN MORE?
- •
- Ask the vaccination provider
- •
- Visit CDC at https://www.cdc.gov/coronavirus/2019-ncov/index.html
- •
- Visit FDA at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
- •
- Contact your state or local public health department
WHERE WILL VACCINATION INFORMATION BE RECORDED?
The vaccination provider may include your or your child’s vaccination information in your state/local jurisdiction’s Immunization Information System (IIS) or other designated system. For more information about IISs, visit: https://www.cdc.gov/vaccines/programs/iis/about.html.
WHAT IS THE COUNTERMEASURES INJURY COMPENSATION PROGRAM?
The Countermeasures Injury Compensation Program (CICP) is a federal program that may help pay for costs of medical care and other specific expenses of certain people who have been seriously injured by certain medicines or vaccines, including this vaccine. Generally, a claim must be submitted to the CICP within one (1) year from the date of receiving the vaccine. To learn more about this program, visit www.hrsa.gov/cicp/ or call 1-855-266-2427.
WHAT IS AN EMERGENCY USE AUTHORIZATION (EUA)?
The FDA has made Novavax COVID-19 Vaccine, Adjuvanted available under an emergency access mechanism called an EUA. An EUA is supported by a Secretary of Health and Human Services (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic. A product authorized for emergency use has not undergone the same type of review by FDA as an FDA-approved product.
FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of the scientific evidence available showing that the product may be effective to prevent COVID-19 during the COVID-19 pandemic and that the known and potential benefits of the product outweigh the known and potential risks of the product. All of these criteria must be met to allow for the product to be used during the COVID-19 pandemic.
The EUA is in effect for the duration of the COVID-19 EUA declaration justifying emergency use of this product, unless terminated or revoked (after which the product may no longer be used).
Manufactured for:
Novavax, Inc., Gaithersburg, MD, 20878
C20101US-00X
Revised: October 3, 2023©2022 Novavax, Inc. All rights reserved.

Scan to capture that this Fact Sheet was provided to vaccine recipient for the electronic medical records/immunization information systems.GDTI: 0886983000370
- 1
- The Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) contains the spike protein of SARS-CoV-2 Omicron variant lineage XBB.1.5 (Omicron XBB.1.5).
- 2
- Monovalent refers to a COVID-19 vaccine that contains or encodes the spike protein of only the Original SARS-CoV-2.
- 3
- Bivalent refers to a COVID-19 vaccine that encodes the spike protein of the Original SARS-CoV-2 and the Omicron BA.4/BA.5 SARS-CoV-2.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 80631-100-10 novavax
Novavax COVID-19 Vaccine,
AdjuvantedSuspension for Intramuscular Injection
10 Multi-Dose Vials
Each vial contains 10 doses of 0.5 mLFor use under Emergency Use Authorization
NDC 80631-100-01
Novavax COVID-19 Vaccine,
AdjuvantedSuspension for Intramuscular Injection
Multi-dose vial (10 doses of 0.5mL)For use under Emergency Use
Authorization. Store at 2 to 8°C
(36 to 46°F). DO NOT FREEZE.
After first puncture, hold at 2 to 25°C
(36 to 77°F). Discard after 12 hours.
No Preservative. Protect from light.20018470/1
Mfd. for: Novavax, Inc.,
Gaithersburg, MD 20878
NDC 80631-102-10 novavax
Novavax COVID-19 Vaccine,
AdjuvantedSuspension for Intramuscular Injection
10 Multi-Dose Vials
Each vial contains 5 doses of 0.5 mLFor use under Emergency Use Authorization
NDC 80631-102-01
Novavax COVID-19 Vaccine,
AdjuvantedSuspension for Intramuscular Injection
Multi-dose vial (5 doses of 0.5mL)For use under Emergency Use
Authorization. Store at 2 to 8°C
(36 to 46°F). DO NOT FREEZE.
After first puncture, hold at 2 to 25°C
(36 to 77°F). Discard after 12 hours.
No Preservative. Protect from light.20018984/1
Mfd. for: Novavax, Inc.,
Gaithersburg, MD 20878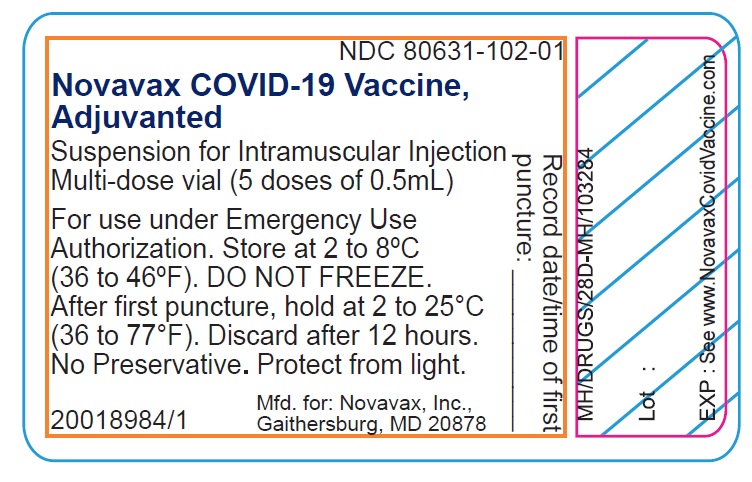
NDC 80631-105-02 novavax
Novavax COVID-19 Vaccine,
Adjuvanted2023-2024 Formula
Suspension for Intramuscular Injection
2 multi-dose vials
Each vial contains 5 doses of 0.5 mLFor 12 years of age and older
For use under Emergency Use Authorization
Rx Only
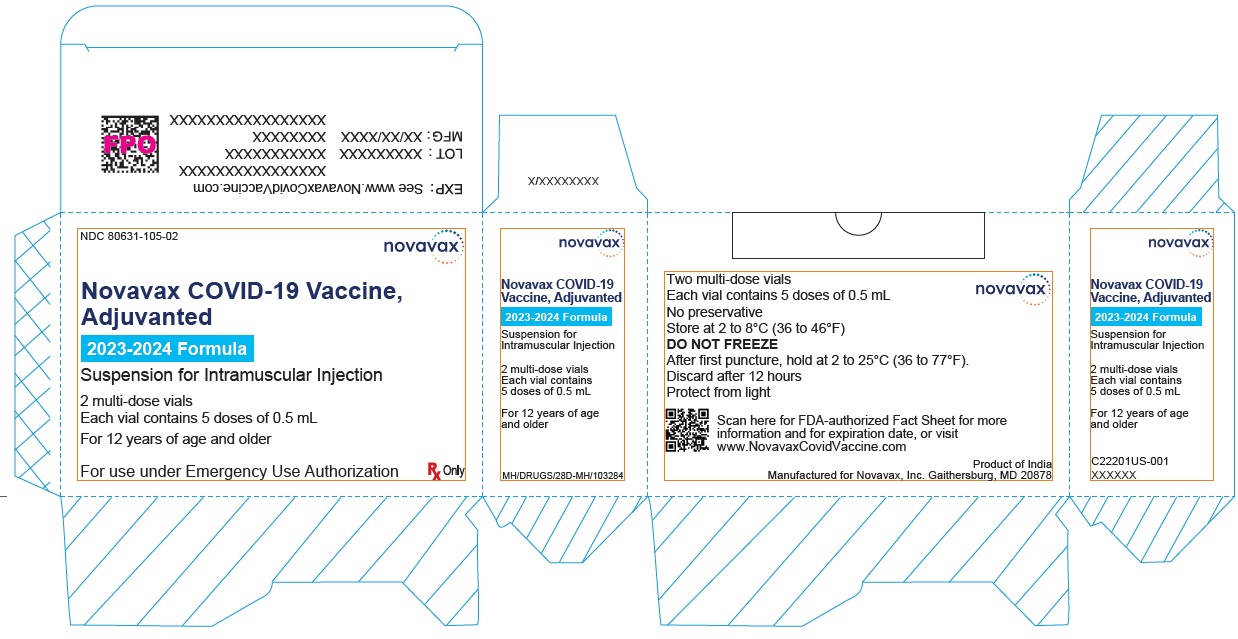
NDC 80631-105-01
Novavax COVID-19 Vaccine,
Adjuvanted2023-2024 Formula
Multi-dose vial (5 doses of 0.5mL)
For IM use. For use under Emergency
Use Authorization. Store at 2 to 8°C
(36 to 46°F). DO NOT FREEZE.
After first puncture, hold at 2 to 25°C
(36 to 77°F). Discard after 12 hours.
No Preservative. Protect from light.Product of India
Mfd. for: Novavax, Inc., Gaithersburg, MD 20878
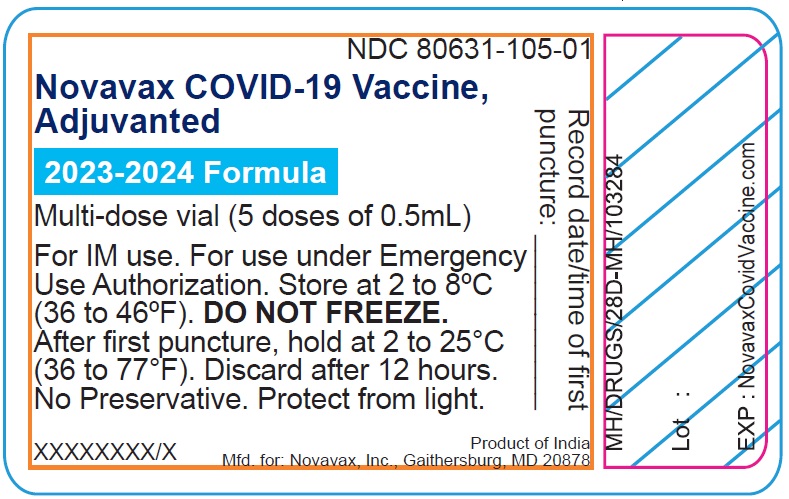
-
INGREDIENTS AND APPEARANCE
NOVAVAX COVID-19 VACCINE, ADJUVANTED
nvx-cov2373 injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:80631-100 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 (UNII: UK9AK2IN1P) (SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 - UNII:UK9AK2IN1P) SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 5 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength CHOLESTEROL (UNII: 97C5T2UQ7J) MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80631-100-10 10 in 1 CARTON 1 NDC:80631-100-01 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date emergency use authorization 01/31/2022 NOVAVAX COVID-19 VACCINE, ADJUVANTED
nvx-cov2373 injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:80631-102 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 (UNII: UK9AK2IN1P) (SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 - UNII:UK9AK2IN1P) SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 5 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength CHOLESTEROL (UNII: 97C5T2UQ7J) MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80631-102-10 10 in 1 CARTON 1 NDC:80631-102-01 2.5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date emergency use authorization 02/01/2023 NOVAVAX COVID-19 VACCINE, ADJUVANTED
nvx-cov2373 injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:80631-105 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 (UNII: UK9AK2IN1P) (SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 - UNII:UK9AK2IN1P) SARS-COV-2 SPIKE GLYCOPROTEIN VACCINE ANTIGEN NVX-COV2373 5 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength CHOLESTEROL (UNII: 97C5T2UQ7J) MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80631-105-02 2 in 1 CARTON 1 NDC:80631-105-01 2.5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date emergency use authorization 10/03/2023 Labeler - Novavax, Inc. (808837520)