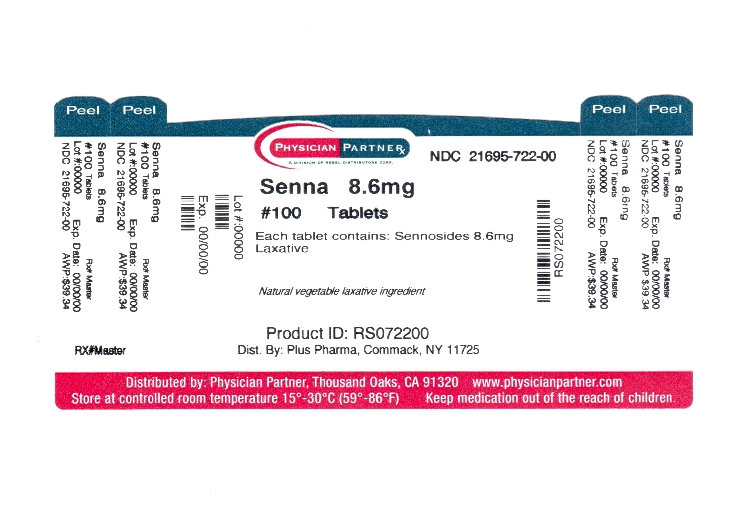
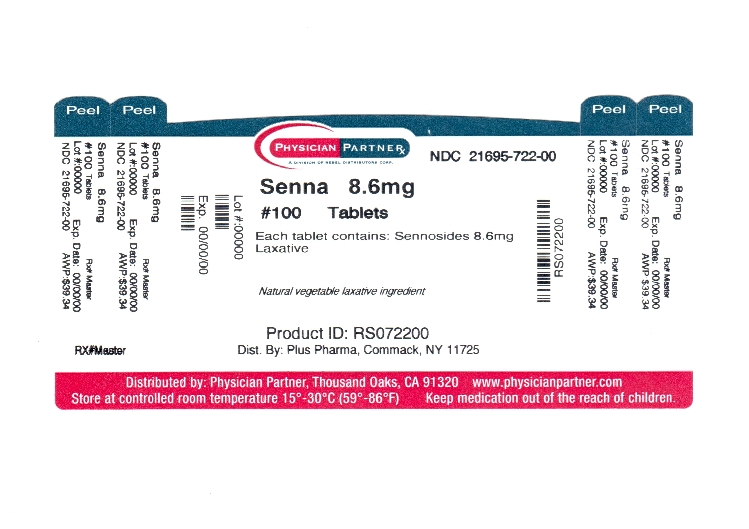
Label: SENNA PLUS tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-722-00, 21695-722-60 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 51645-851
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 7, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Uses
-
Warnings
Do not use ° for longer than one week ° when abdominal pain, nausea or vomiting are present.
Ask a doctor or pharmacist before use if you have noticed a sudden change in bowel habits that lasts over a period of two weeks.
Stop use and ask a doctor if ° you have rectal bleeding ° you fail to have a bowel movement after use of this product, this may indicate a serious condition.
If pregnant or breast-feeding, ask a health care professional before use.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
° Take preferably at bedtime or as directed by a doctor ° if you do not have a comfortable bowel movement by the second day, increase dose by one table (do not exceed the maximum dosage) or decrease dose until you are comfortable.
Adults & children over 12 years: 2 tablets once a day (maximum dosage, 4 tablets twice a day)
Children 6 to under 12 years: 1 tablet once a day (maximum dosage, 2 tablets twice a day)
Children 2 to under 6 years: 1.2 tablet once a day (maximum dosage, 1 tablet twice a day)
Children under 2 years: do not use
- Other Information
-
Inactive ingredients
Croscarmellose Sodium, Dicalcium Phosphate, FD&C Yellow No. 5, FD&C Yellow No. 6, Hypromellose, Magnesium Silicate, Magnesium Stearate, Microcrystalline Cellulose, Mineral Oil, Polyethylene Glycol, Sodium Benzoate, Starch, Stearic Acid and Titanium Dioxide.
Distributed by: Plus Pharma
Commack, NY 11725
Repackaged by: Rebel Distributors Corp
Thousand Oaks, CA 91320
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SENNA PLUS
senna plus tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21695-722(NDC:51645-851) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES A AND B (UNII: 1B5FPI42EN) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES A AND B 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM SILICATE (UNII: 9B9691B2N9) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) SODIUM BENZOATE (UNII: OJ245FE5EU) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE Score no score Shape ROUND Size 10mm Flavor Imprint Code CPC490 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-722-60 60 in 1 BOTTLE 2 NDC:21695-722-00 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 07/10/2009 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK