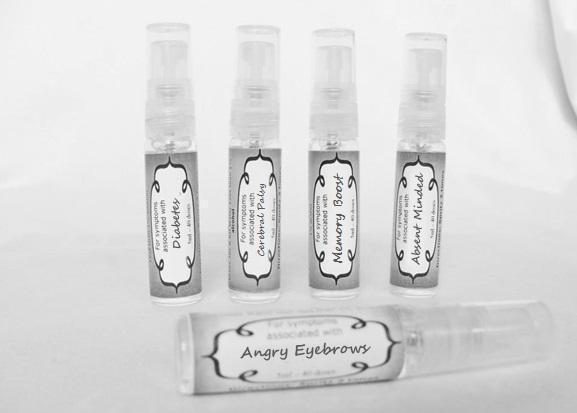
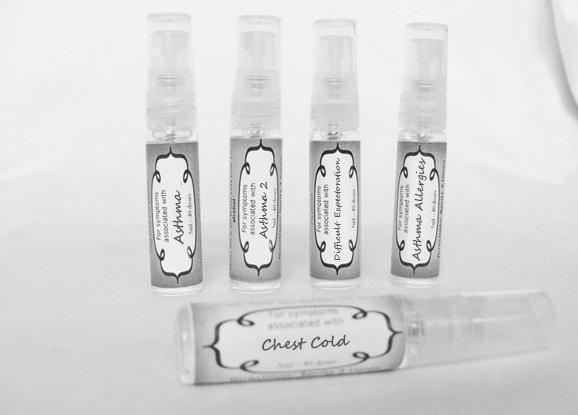
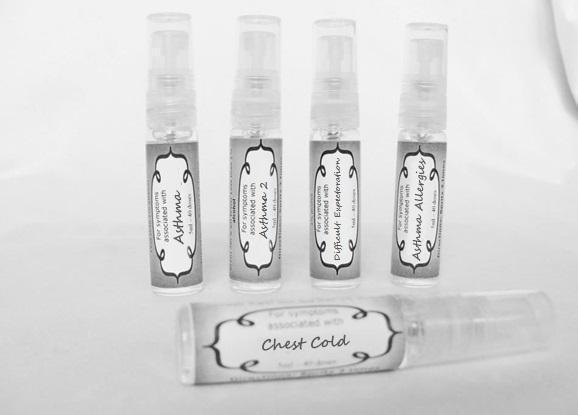
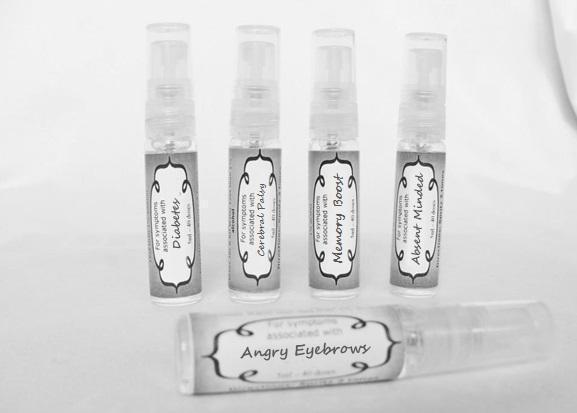
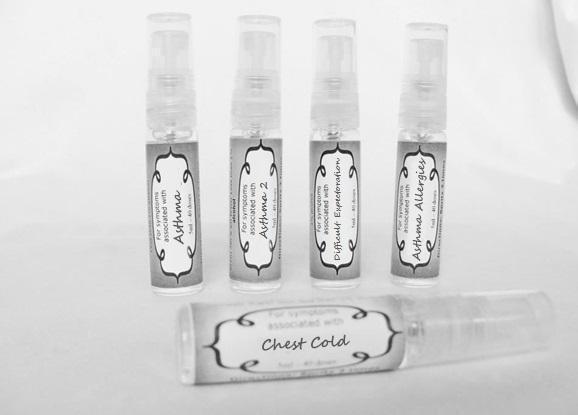
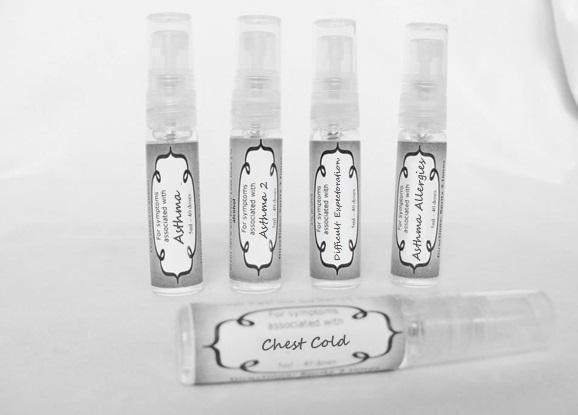
Label: ASTHMA- abies canadensis 30c, adrenalinum 30c, ammi visnaga 30c, cat hair 30c, eriodictyon 30c, eucalyptus glob 30c, human dandruff 30c, house dust mites 30c, okoubaka 30c, origanum 30c, melissa officionalis 30c myrtus communis 30c mentha pepperita 30c, pinus sylvestris. 30c, spray
WHOOPING COUGH 2- aconite 30c, ambrosia 30c, castanea sativa 30c, chelidonium 30c, cuprum aceticum 30c, dulcamara ....... moschata 30c, sulphur 30c spray
STYE IN THE EYE- calc fluor 30c,, conium mac 30c,, carboneum sulph 30c, digitalis 30c, graphites 30c, lycopodium 30c, pulsatilla 30c, sepia 30c, silicea 30c, staphysagria 30c, sulphur 30c spray
WEIGHT LOSS- ambra grisea 30c, antimonium crudum 30c, calc carb 30c, capsicum 30c, ferrum met 30c, graphites 30c, kali carb 30c, phytolacca 30c, sepia spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 59667-0089-2, 59667-0090-2, 59667-0091-2, 59667-0092-2, view more59667-0093-2, 59667-0094-2, 59667-0095-2, 59667-0096-2, 59667-0098-2, 59667-0099-2, 59667-0100-2, 59667-0101-2, 59667-0102-2, 59667-0103-2, 59667-0104-2, 59667-0105-2, 59667-0106-2, 59667-0107-2, 59667-0108-2, 59667-0109-2, 59667-0110-2 - Packager: Home Sweet Homeopathics
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 2, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
- PURPOSE
- INDICATIONS & USAGE
- Directions:
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- Active ingredients:
- Inactive Ingredients:
-
INGREDIENTS AND APPEARANCE
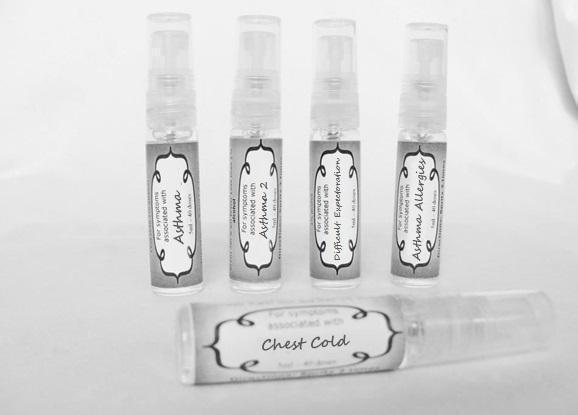
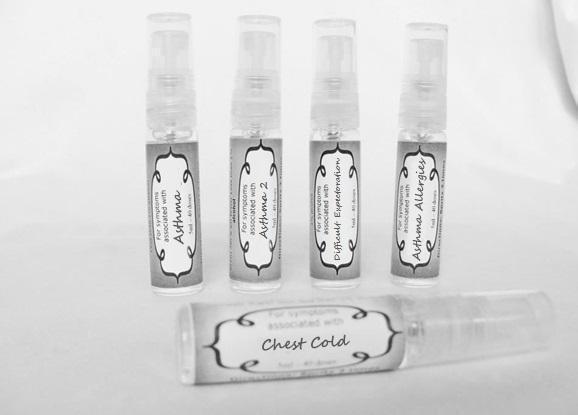
ASTHMA
abies canadensis 30c, adrenalinum 30c, ammi visnaga 30c, cat hair 30c, eriodictyon 30c, eucalyptus glob 30c, human dandruff 30c, house dust mites 30c, okoubaka 30c, origanum 30c, melissa officionalis 30c myrtus communis 30c mentha pepperita 30c, pinus sylvestris. 30c, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0089 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TSUGA CANADENSIS BARK (UNII: R53V5F950S) (TSUGA CANADENSIS BARK - UNII:R53V5F950S) TSUGA CANADENSIS BARK 30 [hp_C] in 30 [hp_C] EPINEPHRINE (UNII: YKH834O4BH) (EPINEPHRINE - UNII:YKH834O4BH) EPINEPHRINE 30 [hp_C] in 30 [hp_C] AMMI VISNAGA FRUIT (UNII: 3UN285QN0C) (AMMI VISNAGA FRUIT - UNII:3UN285QN0C) AMMI VISNAGA FRUIT 30 [hp_C] in 30 [hp_C] FELIS CATUS HAIR (UNII: 1564HD0N96) (FELIS CATUS HAIR - UNII:1564HD0N96) FELIS CATUS HAIR 30 [hp_C] in 30 [hp_C] ERIODICTYON CALIFORNICUM LEAF (UNII: 2Y7TIQ135H) (ERIODICTYON CALIFORNICUM LEAF - UNII:2Y7TIQ135H) ERIODICTYON CALIFORNICUM LEAF 30 [hp_C] in 30 [hp_C] EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 30 [hp_C] in 30 [hp_C] HUMAN DANDER (UNII: 30X630U81C) (HUMAN DANDER - UNII:30X630U81C) HUMAN DANDER 30 [hp_C] in 30 [hp_C] DERMATOPHAGOIDES FARINAE (UNII: PR9U2YPF3Q) (DERMATOPHAGOIDES FARINAE - UNII:PR9U2YPF3Q) DERMATOPHAGOIDES FARINAE 30 [hp_C] in 30 [hp_C] DERMATOPHAGOIDES PTERONYSSINUS (UNII: 57L1Z5378K) (DERMATOPHAGOIDES PTERONYSSINUS - UNII:57L1Z5378K) DERMATOPHAGOIDES PTERONYSSINUS 30 [hp_C] in 30 [hp_C] OKOUBAKA AUBREVILLEI BARK (UNII: MK2074187Z) (OKOUBAKA AUBREVILLEI BARK - UNII:MK2074187Z) OKOUBAKA AUBREVILLEI BARK 30 [hp_C] in 30 [hp_C] ORIGANUM MAJORANA WHOLE (UNII: R40XM3HU5X) (ORIGANUM MAJORANA WHOLE - UNII:R40XM3HU5X) ORIGANUM MAJORANA WHOLE 30 [hp_C] in 30 [hp_C] MELISSA OFFICINALIS (UNII: YF70189L0N) (MELISSA OFFICINALIS - UNII:YF70189L0N) MELISSA OFFICINALIS 30 [hp_C] in 30 [hp_C] MYRTUS COMMUNIS TOP (UNII: 367E55FXGW) (MYRTUS COMMUNIS TOP - UNII:367E55FXGW) MYRTUS COMMUNIS TOP 30 [hp_C] in 30 [hp_C] MENTHA PIPERITA (UNII: 79M2M2UDA9) (MENTHA PIPERITA - UNII:79M2M2UDA9) MENTHA PIPERITA 30 [hp_C] in 30 [hp_C] PINUS SYLVESTRIS LEAFY TWIG (UNII: Q1RGP4UB73) (PINUS SYLVESTRIS LEAFY TWIG - UNII:Q1RGP4UB73) PINUS SYLVESTRIS LEAFY TWIG 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0089-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 WHOOPING COUGH 2
aconite 30c, ambrosia 30c, castanea sativa 30c, chelidonium 30c, cuprum aceticum 30c, dulcamara 30c, hepar sulph 30c, hydrocyanicum 30c, hyoscyamus 30c, kali bich 30c kali mur 30c lobelia30c, mag phos. 30c, naphtha 30c, sepia 30c, trifolium 30c, justicia 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0090 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 30 [hp_C] in 30 [hp_C] AMBROSIA ARTEMISIIFOLIA (UNII: 9W34L2CQ9A) (AMBROSIA ARTEMISIIFOLIA - UNII:9W34L2CQ9A) AMBROSIA ARTEMISIIFOLIA 30 [hp_C] in 30 [hp_C] CASTANEA SATIVA FLOWER (UNII: YHZ719F7M3) (CASTANEA SATIVA FLOWER - UNII:YHZ719F7M3) CASTANEA SATIVA FLOWER 30 [hp_C] in 30 [hp_C] CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 30 [hp_C] in 30 [hp_C] CUPRIC ACETATE (UNII: 39M11XPH03) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 30 [hp_C] in 30 [hp_C] SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] HYDROGEN CYANIDE (UNII: 2WTB3V159F) (HYDROGEN CYANIDE - UNII:2WTB3V159F) HYDROGEN CYANIDE 30 [hp_C] in 30 [hp_C] HYOSCYAMUS NIGER (UNII: 4WRK2153H3) (HYOSCYAMUS NIGER - UNII:4WRK2153H3) HYOSCYAMUS NIGER 30 [hp_C] in 30 [hp_C] POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 30 [hp_C] in 30 [hp_C] POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 30 [hp_C] in 30 [hp_C] LOBELIA INFLATA (UNII: 9PP1T3TC5U) (LOBELIA INFLATA - UNII:9PP1T3TC5U) LOBELIA INFLATA 30 [hp_C] in 30 [hp_C] MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 30 [hp_C] in 30 [hp_C] NAPHTHALENE (UNII: 2166IN72UN) (NAPHTHALENE - UNII:2166IN72UN) NAPHTHALENE 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) (TRIFOLIUM PRATENSE FLOWER - UNII:4JS0838828) TRIFOLIUM PRATENSE FLOWER 30 [hp_C] in 30 [hp_C] JUSTICIA ADHATODA LEAF (UNII: HH159XOV81) (JUSTICIA ADHATODA LEAF - UNII:HH159XOV81) JUSTICIA ADHATODA LEAF 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0090-2 30 [hp_C] in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 DIFFICULT EXPECTORATION
antimonium tart 30c, arsenicum 30c, belladonna 30c, bacillinum 200c, calcarea carb 30c, cactus 30c, causticum 30c, cuprum met. 30c, dulcamara 30c, ipecac 30c, hepar sulph 30c, kali sulph 30c, lycopodium 30c, natrum carb 30c, nux vomica 30c, phosphorus 30c, pulsatilla 30c, sepia 30c, senega 30c, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0091 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 30 [hp_C] in 30 [hp_C] ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] in 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] ESCHERICHIA COLI (UNII: 514B9K0L10) (ESCHERICHIA COLI - UNII:514B9K0L10) ESCHERICHIA COLI 200 [hp_C] in 30 [hp_C] OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_C] in 30 [hp_C] SELENICEREUS GRANDIFLORUS STEM (UNII: 7114SV0MYK) (SELENICEREUS GRANDIFLORUS STEM - UNII:7114SV0MYK) SELENICEREUS GRANDIFLORUS STEM 30 [hp_C] in 30 [hp_C] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] in 30 [hp_C] COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 30 [hp_C] in 30 [hp_C] SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 30 [hp_C] in 30 [hp_C] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 30 [hp_C] in 30 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 30 [hp_C] SODIUM CARBONATE (UNII: 45P3261C7T) (CARBONATE ION - UNII:7UJQ5OPE7D) SODIUM CARBONATE 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) (POLYGALA SENEGA ROOT - UNII:M7T6H7D4IF) POLYGALA SENEGA ROOT 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0091-2 30 [hp_C] in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 CHEST COLD
antimonium tart 30c, bacillinum 200c, bryonia 30c, camphora 30c, chelidonoium 30c, grindelia 30c, kreosotum 30c, lycopodium 30c, natrum sulph 30c, phosphorous 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0092 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 30 [hp_C] in 30 [hp_C] ESCHERICHIA COLI (UNII: 514B9K0L10) (ESCHERICHIA COLI - UNII:514B9K0L10) ESCHERICHIA COLI 200 [hp_C] in 30 [hp_C] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_C] in 30 [hp_C] CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 30 [hp_C] in 30 [hp_C] CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 30 [hp_C] in 30 [hp_C] GRINDELIA HIRSUTULA FLOWERING TOP (UNII: IDB0NAZ6AI) (GRINDELIA HIRSUTULA FLOWERING TOP - UNII:IDB0NAZ6AI) GRINDELIA HIRSUTULA FLOWERING TOP 30 [hp_C] in 30 [hp_C] WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 30 [hp_C] in 30 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 30 [hp_C] SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0092-2 30 [hp_C] in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 BRONCHITIS
aesculos 30c, antimonium tart 30c, arsenicum 30c, baryta mur 30c, bryonia 30c, causticum 30c, dulcamara 30c, hepar sulph. 30c, phosphorous 30c, sanguinaria 30c , senicio 30c, stannum met 30c, sulphur 30c, quebracho 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0093 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 30 [hp_C] in 30 [hp_C] ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 30 [hp_C] in 30 [hp_C] ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] in 30 [hp_C] BARIUM CHLORIDE DIHYDRATE (UNII: EL5GJ3U77E) (BARIUM CATION - UNII:V645272HLN) BARIUM CHLORIDE DIHYDRATE 30 [hp_C] in 30 [hp_C] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_C] in 30 [hp_C] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] in 30 [hp_C] SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] SANGUINARIA CANADENSIS ROOT (UNII: N9288CD508) (SANGUINARIA CANADENSIS ROOT - UNII:N9288CD508) SANGUINARIA CANADENSIS ROOT 30 [hp_C] in 30 [hp_C] SENECIO VULGARIS (UNII: W6MU8IYV12) (SENECIO VULGARIS - UNII:W6MU8IYV12) SENECIO VULGARIS 30 [hp_C] in 30 [hp_C] TIN (UNII: 387GMG9FH5) (TIN - UNII:387GMG9FH5) TIN 30 [hp_C] in 30 [hp_C] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] in 30 [hp_C] ASPIDOSPERMA QUEBRACHO-BLANCO BARK (UNII: 52B1340190) (ASPIDOSPERMA QUEBRACHO-BLANCO BARK - UNII:52B1340190) ASPIDOSPERMA QUEBRACHO-BLANCO BARK 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0093-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 WHOOPING COUGH
antimonium tart 30c, belladonna 30c, carbo veg 30c, cina 30c, coccus cacti 30c, cuprum met 30c, dioscorea 30c, drosera 30c, formalinum 30c, ignatia 30c, ipecac 30c, kali brom 30c, kali carb 30c, kali sulph 30c, mephitis 30c, nux vomica 30c pertussinum 30c, pulsatilla 30c veratrum 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0094 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 30 [hp_C] in 30 [hp_C] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_C] in 30 [hp_C] ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 30 [hp_C] in 30 [hp_C] ARTEMISIA CINA PRE-FLOWERING TOP (UNII: 28M1820ACT) (ARTEMISIA CINA FLOWER - UNII:28M1820ACT) ARTEMISIA CINA PRE-FLOWERING TOP 30 [hp_C] in 30 [hp_C] PROTORTONIA CACTI (UNII: LZB7TFX1LT) (PROTORTONIA CACTI - UNII:LZB7TFX1LT) PROTORTONIA CACTI 30 [hp_C] in 30 [hp_C] COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 30 [hp_C] in 30 [hp_C] DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) (DIOSCOREA VILLOSA ROOT - UNII:IWY3IWX2G8) DIOSCOREA VILLOSA TUBER 30 [hp_C] in 30 [hp_C] DROSERA ROTUNDIFOLIA (UNII: QR44N9XPJQ) (DROSERA ROTUNDIFOLIA - UNII:QR44N9XPJQ) DROSERA ROTUNDIFOLIA 30 [hp_C] in 30 [hp_C] FORMALDEHYDE (UNII: 1HG84L3525) (FORMALDEHYDE - UNII:1HG84L3525) FORMALDEHYDE 30 [hp_C] in 30 [hp_C] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] in 30 [hp_C] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 30 [hp_C] in 30 [hp_C] POTASSIUM BROMIDE (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) POTASSIUM BROMIDE 30 [hp_C] in 30 [hp_C] POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 30 [hp_C] in 30 [hp_C] POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 30 [hp_C] in 30 [hp_C] MEPHITIS MEPHITIS ANAL GLAND FLUID (UNII: 3BN57UN4US) (MEPHITIS MEPHITIS ANAL GLAND FLUID - UNII:3BN57UN4US) MEPHITIS MEPHITIS ANAL GLAND FLUID 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] HUMAN SPUTUM, BORDETELLA PERTUSSIS INFECTED (UNII: U364V64HUN) (HUMAN SPUTUM, BORDETELLA PERTUSSIS INFECTED - UNII:U364V64HUN) HUMAN SPUTUM, BORDETELLA PERTUSSIS INFECTED 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] VERATRUM ALBUM ROOT (UNII: QNS6W5US1Z) (VERATRUM ALBUM ROOT - UNII:QNS6W5US1Z) VERATRUM ALBUM ROOT 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0094-2 30 [hp_C] in 1 BOTTLE 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 WET WEATHER ASTHMA
arnica 30c, belladonna 30c,hyoscyamus 30c, lachesis 30c, nux moschata 30c, silicea 30c, sepia 30c, stramonium 30c, sulphur 30c, veratrum album 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0095 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] HYOSCYAMUS NIGER (UNII: 4WRK2153H3) (HYOSCYAMUS NIGER - UNII:4WRK2153H3) HYOSCYAMUS NIGER 30 [hp_C] in 30 [hp_C] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_C] in 30 [hp_C] NUTMEG (UNII: AEE24M3MQ9) (NUTMEG - UNII:AEE24M3MQ9) NUTMEG 30 [hp_C] in 30 [hp_C] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] DATURA STRAMONIUM (UNII: G6W4F0V8Z3) (DATURA STRAMONIUM - UNII:G6W4F0V8Z3) DATURA STRAMONIUM 30 [hp_C] in 30 [hp_C] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] in 30 [hp_C] VERATRUM ALBUM ROOT (UNII: QNS6W5US1Z) (VERATRUM ALBUM ROOT - UNII:QNS6W5US1Z) VERATRUM ALBUM ROOT 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0095-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 ASTHMA ALLERGIES
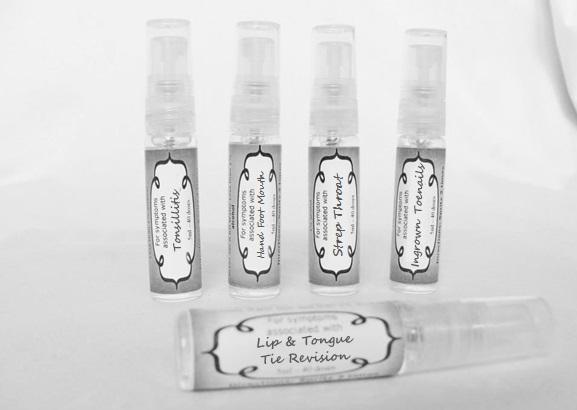
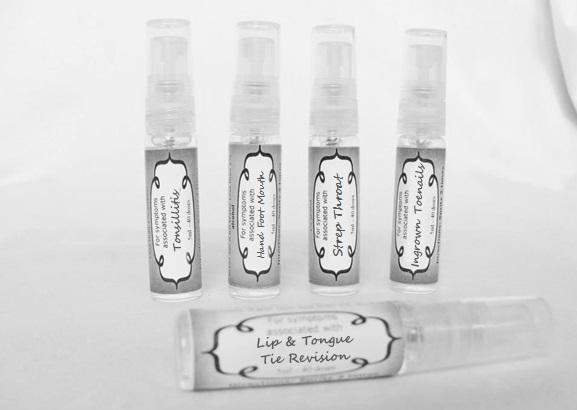
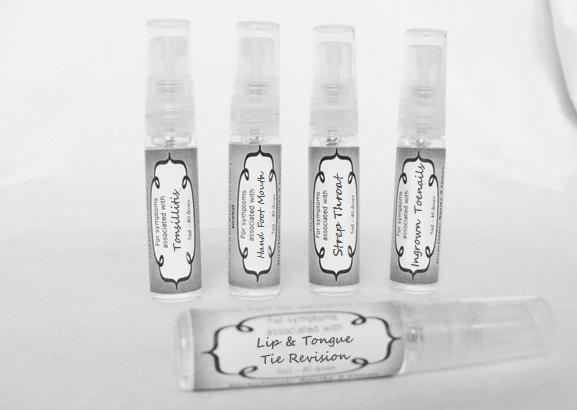
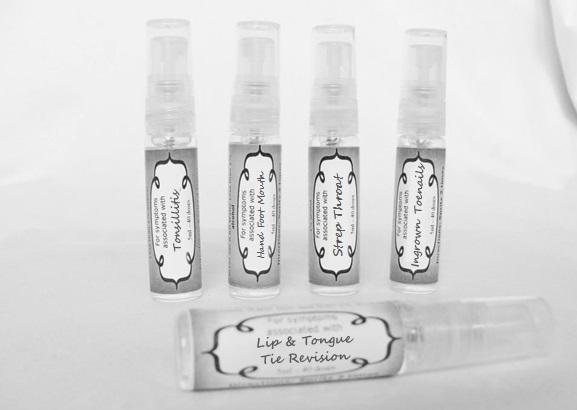
allium cepa 30c, arsenicum 30c, thuja 30c, badiaga 30c, carbo veg 30c, dulcamara 30c, euphrasia 30c, iodatum 30c, naja 30c, nat sulph 30c, nux vomica 30c, sabadilla 30c, sinapis nigra 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0096 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 30 [hp_C] in 30 [hp_C] ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] in 30 [hp_C] SPONGILLA LACUSTRIS (UNII: 6SZ0G98BHM) (SPONGILLA LACUSTRIS - UNII:6SZ0G98BHM) SPONGILLA LACUSTRIS 30 [hp_C] in 30 [hp_C] THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 30 [hp_C] in 30 [hp_C] ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 30 [hp_C] in 30 [hp_C] SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 30 [hp_C] in 30 [hp_C] EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 30 [hp_C] in 30 [hp_C] IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 30 [hp_C] in 30 [hp_C] NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM 30 [hp_C] in 30 [hp_C] SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 30 [hp_C] in 30 [hp_C] BLACK MUSTARD SEED (UNII: 8LTY55LQ8D) (BLACK MUSTARD SEED - UNII:8LTY55LQ8D) BLACK MUSTARD SEED 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0096-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 STREP THROAT
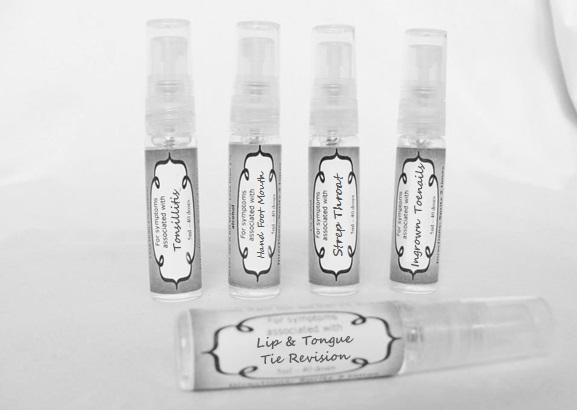
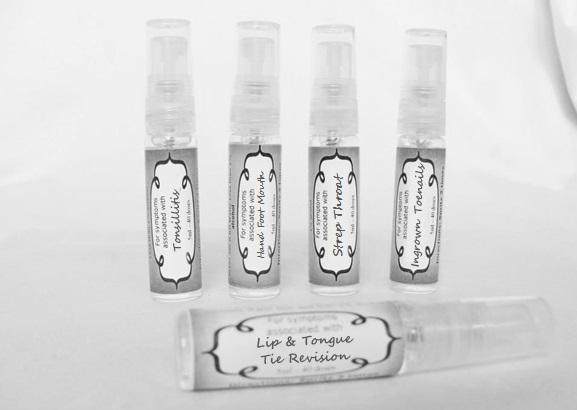
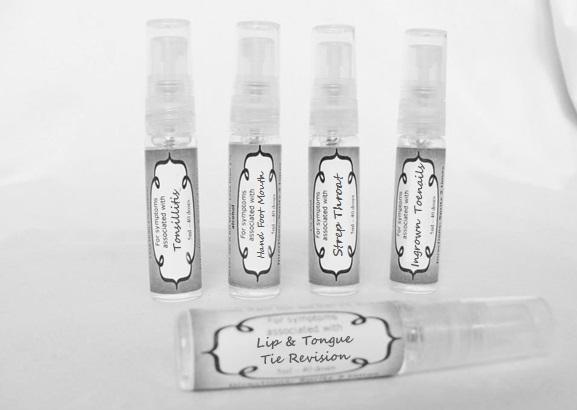
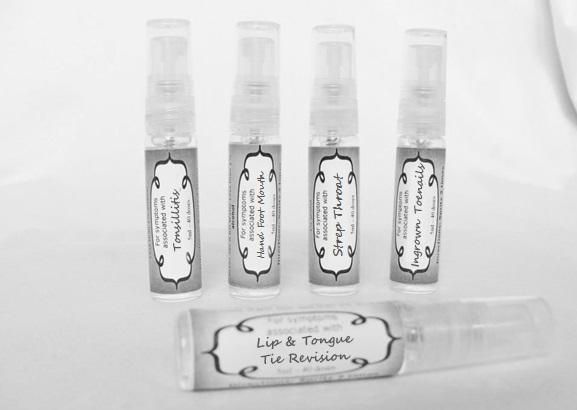
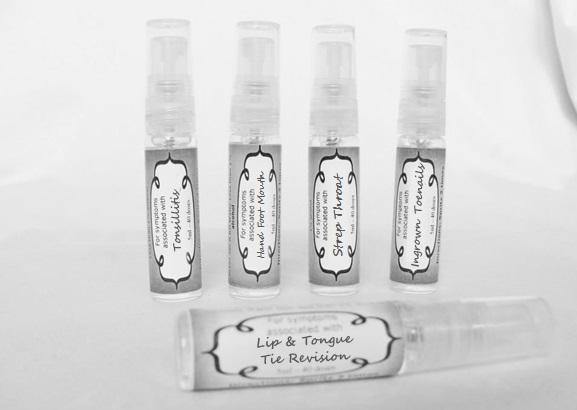
arum tri 30c, belledonna 30c, carbo animalis 30c, causticum 30c, echinacea30c, hydrastis 30c, lachesis 30c, phosphorous 30c, phytolacca 30c, nitricum acidum 30c, nux vomica 30c, mercurius sol 30c , streptococcinum 30c, sabadilla 30c, spigellia 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0098 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARISAEMA TRIPHYLLUM ROOT (UNII: DM64K844DM) (ARISAEMA TRIPHYLLUM ROOT - UNII:DM64K844DM) ARISAEMA TRIPHYLLUM ROOT 30 [hp_C] in 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] CARBO ANIMALIS (UNII: 279O8I0433) (CARBO ANIMALIS - UNII:279O8I0433) CARBO ANIMALIS 30 [hp_C] in 30 [hp_C] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] in 30 [hp_C] ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 30 [hp_C] in 30 [hp_C] GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 30 [hp_C] in 30 [hp_C] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 30 [hp_C] in 30 [hp_C] NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 30 [hp_C] in 30 [hp_C] STREPTOCOCCUS PYOGENES (UNII: LJ2LP0YL98) (STREPTOCOCCUS PYOGENES - UNII:LJ2LP0YL98) STREPTOCOCCUS PYOGENES 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 30 [hp_C] in 30 [hp_C] SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 30 [hp_C] in 30 [hp_C] SPIGELIA ANTHELMIA (UNII: WYT05213GE) (SPIGELIA ANTHELMIA - UNII:WYT05213GE) SPIGELIA ANTHELMIA 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0098-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 LIP AND TONGUE TIE REVISION
artemisia30c, calendula 30c, carbolicum acidum 30c, hypericum 30c, staphysagria 30c, sulphuricum acidum 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0099 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARTEMISIA ABROTANUM FLOWERING TOP (UNII: QG07G580U0) (ARTEMISIA ABROTANUM FLOWERING TOP - UNII:QG07G580U0) ARTEMISIA ABROTANUM FLOWERING TOP 30 [hp_C] in 30 [hp_C] CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 30 [hp_C] in 30 [hp_C] PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 30 [hp_C] in 30 [hp_C] HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 30 [hp_C] in 30 [hp_C] DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 30 [hp_C] in 30 [hp_C] SULFURIC ACID (UNII: O40UQP6WCF) (SULFURIC ACID - UNII:O40UQP6WCF) SULFURIC ACID 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0099-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 TONSILLITIS
alumen 30c, baryta carb 30c, baryta mur 30c, belladonna 30c, ferrum phos 30c guiaicum 30c, hepar sulph 30c, lac caninum 30c, lachesis 30c, merc sol 30c, merc dulcies 30c, nitricum acidum 30c, silicea 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUM, POTASSIUM (UNII: 1L24V9R23S) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUM, POTASSIUM 30 [hp_C] in 30 [hp_C] BARIUM CHLORIDE DIHYDRATE (UNII: EL5GJ3U77E) (BARIUM CATION - UNII:V645272HLN) BARIUM CHLORIDE DIHYDRATE 30 [hp_C] in 30 [hp_C] BARIUM CARBONATE (UNII: 6P669D8HQ8) (BARIUM CATION - UNII:V645272HLN) BARIUM CARBONATE 30 [hp_C] in 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 30 [hp_C] in 30 [hp_C] GUAIAC (UNII: 03C8A0DFJ8) (GUAIAC - UNII:03C8A0DFJ8) GUAIAC 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] CANIS LUPUS FAMILIARIS MILK (UNII: G39P120JQT) (CANIS LUPUS FAMILIARIS MILK - UNII:G39P120JQT) CANIS LUPUS FAMILIARIS MILK 30 [hp_C] in 30 [hp_C] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_C] in 30 [hp_C] MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 30 [hp_C] in 30 [hp_C] CALOMEL (UNII: J2D46N657D) (CALOMEL - UNII:J2D46N657D) CALOMEL 30 [hp_C] in 30 [hp_C] NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 30 [hp_C] in 30 [hp_C] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0100-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 HAND FOOT MOUTH
antimonium crudum 30c, argentum nit 30c, borax 30c, kali chloricum 30c, mercurius corr 30c, nat mur 30c, nitricum ac 30c, sulphuricum acidum 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 30 [hp_C] in 30 [hp_C] SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 30 [hp_C] in 30 [hp_C] SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 30 [hp_C] in 30 [hp_C] POTASSIUM CHLORATE (UNII: H35KS68EE7) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORATE 30 [hp_C] in 30 [hp_C] MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 30 [hp_C] in 30 [hp_C] SULFURIC ACID (UNII: O40UQP6WCF) (SULFURIC ACID - UNII:O40UQP6WCF) SULFURIC ACID 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0101-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 INGROWN TOENAILS
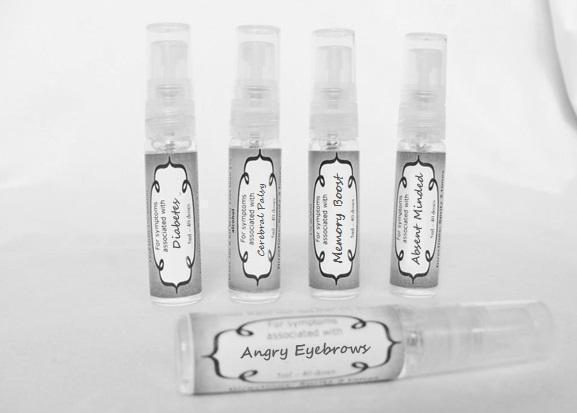
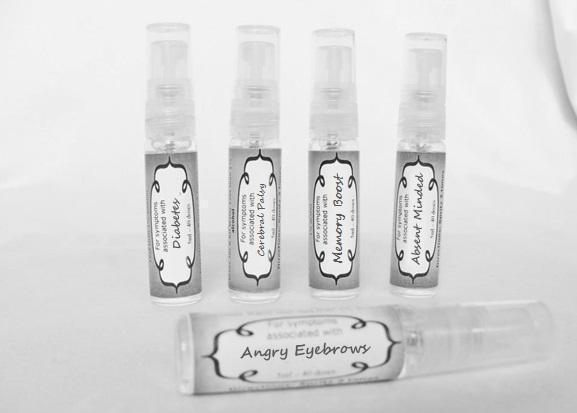
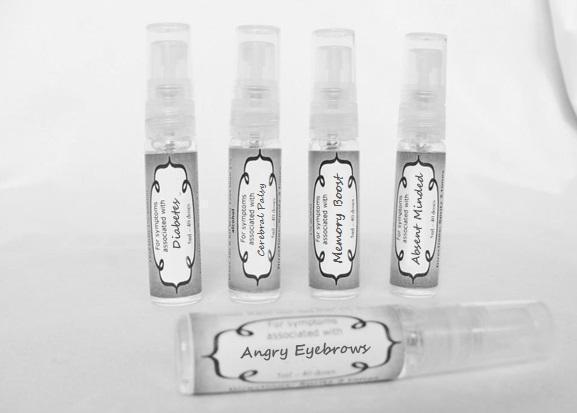
causticum 30c, graphites 30c, hepar sulph 30c, mag phos 30c, magnetis polus australis 30c, nat mur 30c, nitricum acidum 30c, phosphoricum ac 30c, silicea 30c, sulphur 30c, thuja 30c, teucrium 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] in 30 [hp_C] GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 30 [hp_C] in 30 [hp_C] CALCIUM SULFATE (UNII: WAT0DDB505) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE 30 [hp_C] in 30 [hp_C] MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 30 [hp_C] in 30 [hp_C] PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 30 [hp_C] in 30 [hp_C] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 30 [hp_C] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] in 30 [hp_C] THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 30 [hp_C] in 30 [hp_C] TEUCRIUM MARUM (UNII: 10464S0TAA) (TEUCRIUM MARUM - UNII:10464S0TAA) TEUCRIUM MARUM 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0102-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 DIABETES
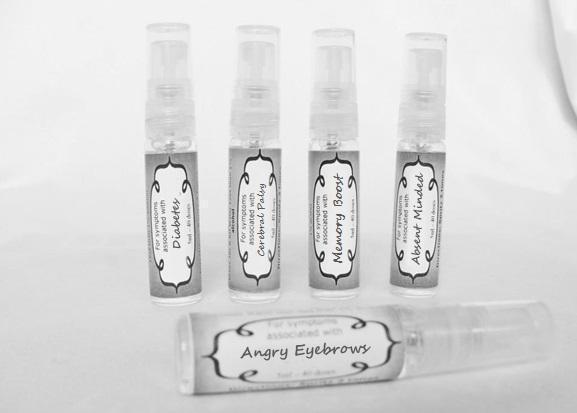
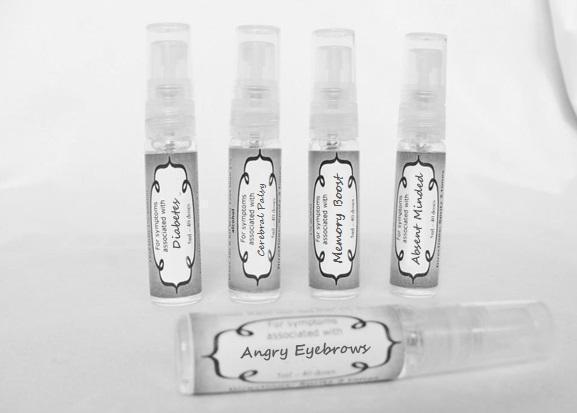
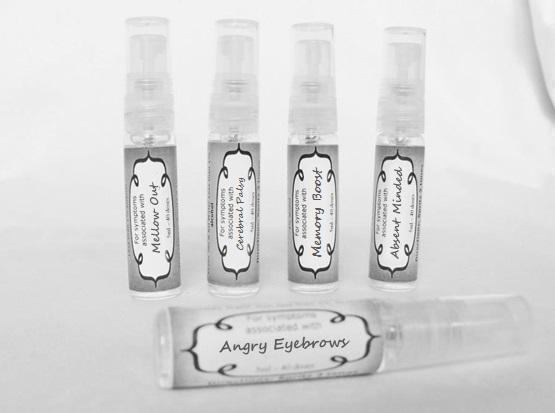
aceticum acidum, alloxanum, bovista, carcinocinum, helonias, lycopodium, phosphoricum acidum, phosphorus, plumbum met, tarentula. syzygium ,terebinthiniae, uranium nit. sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0103 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 30 [hp_C] in 30 [hp_C] ALLOXAN (UNII: 6SW5YHA5NG) (ALLOXAN - UNII:6SW5YHA5NG) ALLOXAN 30 [hp_C] in 30 [hp_C] GIANT PUFFBALL (UNII: I6839Y031A) (GIANT PUFFBALL - UNII:I6839Y031A) GIANT PUFFBALL 30 [hp_C] in 30 [hp_C] HUMAN BREAST TUMOR CELL (UNII: C62OO7VD9K) (HUMAN BREAST TUMOR CELL - UNII:C62OO7VD9K) HUMAN BREAST TUMOR CELL 30 [hp_C] in 30 [hp_C] CHAMAELIRIUM LUTEUM ROOT (UNII: DQV54Y5H3U) (CHAMAELIRIUM LUTEUM ROOT - UNII:DQV54Y5H3U) CHAMAELIRIUM LUTEUM ROOT 30 [hp_C] in 30 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 30 [hp_C] PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 30 [hp_C] in 30 [hp_C] LYCOSA TARANTULA (UNII: 86M454L2TT) (LYCOSA TARANTULA - UNII:86M454L2TT) LYCOSA TARANTULA 30 [hp_C] in 30 [hp_C] SYZYGIUM CUMINI SEED (UNII: 820LSF646I) (SYZYGIUM CUMINI SEED - UNII:820LSF646I) SYZYGIUM CUMINI SEED 30 [hp_C] in 30 [hp_C] TURPENTINE OIL (UNII: C5H0QJ6V7F) (TURPENTINE OIL - UNII:C5H0QJ6V7F) TURPENTINE OIL 30 [hp_C] in 30 [hp_C] URANYL NITRATE HEXAHYDRATE (UNII: 3V057702FY) (URANIUM CATION (6+) - UNII:5PI36AS4G7) URANYL NITRATE HEXAHYDRATE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0103-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 CEREBRAL PALSY
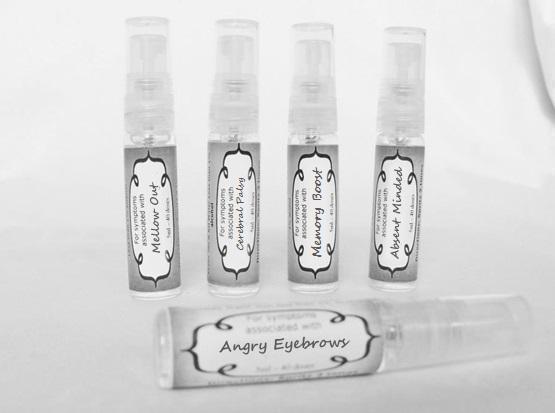
arnica 30c, causticum 30c, conium mac 30c, gelsemium 30c, helleborus 30c, ignatia 30c, lathyrus 30c, opium 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) (ARNICA MONTANA FLOWER - UNII:OZ0E5Y15PZ) ARNICA MONTANA FLOWER 30 [hp_C] in 30 [hp_C] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] in 30 [hp_C] CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 30 [hp_C] in 30 [hp_C] GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 30 [hp_C] in 30 [hp_C] HELLEBORUS NIGER ROOT (UNII: 608DGJ6815) (HELLEBORUS NIGER ROOT - UNII:608DGJ6815) HELLEBORUS NIGER ROOT 30 [hp_C] in 30 [hp_C] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] in 30 [hp_C] LATHYRUS SATIVAS SEED (UNII: 8VP54WOT4I) (LATHYRUS SATIVAS SEED - UNII:8VP54WOT4I) LATHYRUS SATIVAS SEED 30 [hp_C] in 30 [hp_C] OPIUM (UNII: 37M3MZ001L) (OPIUM - UNII:37M3MZ001L) OPIUM 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0104-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 ANGRY EYEBROWS
aconite 30c, anacardium 30c, arsenicum 30c, aurum met. 30c, bryonia 30c, chamomilla 30c, hepar sulph 30c, ignatia 30c, kali sulph 30c, lycopodium 30c, merc sol 30c, nat mur 30c, nitricum acidum 30c, nux vomica 30c, petroleum 30c, sepia 30c, staphysagria 30c, sulphur 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS ROOT (UNII: KPD2N7348X) (ACONITUM NAPELLUS ROOT - UNII:KPD2N7348X) ACONITUM NAPELLUS ROOT 30 [hp_C] in 30 [hp_C] SEMECARPUS ANACARDIUM JUICE (UNII: Y0F0BU8RDU) (SEMECARPUS ANACARDIUM JUICE - UNII:Y0F0BU8RDU) SEMECARPUS ANACARDIUM JUICE 30 [hp_C] in 30 [hp_C] ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] in 30 [hp_C] GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 30 [hp_C] in 30 [hp_C] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] in 30 [hp_C] POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 30 [hp_C] in 30 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 30 [hp_C] MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] KEROSENE (UNII: 1C89KKC04E) (KEROSENE - UNII:1C89KKC04E) KEROSENE 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] in 30 [hp_C] DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0105-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 MELLOW OUT
arsenicum 30c, chamomilla 30c, cina 30c, glononium 30c, hyoscyamus 30c, medorrhinum 30c, stramonium 30c, tarentula 30c, veratrum album 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] ARTEMISIA CINA PRE-FLOWERING TOP (UNII: 28M1820ACT) (ARTEMISIA CINA FLOWER - UNII:28M1820ACT) ARTEMISIA CINA PRE-FLOWERING TOP 30 [hp_C] in 30 [hp_C] NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 30 [hp_C] in 30 [hp_C] HYOSCYAMUS NIGER (UNII: 4WRK2153H3) (HYOSCYAMUS NIGER - UNII:4WRK2153H3) HYOSCYAMUS NIGER 30 [hp_C] in 30 [hp_C] GONORRHEAL URETHRAL SECRETION HUMAN (UNII: 9BZG9E3I8F) (GONORRHEAL URETHRAL SECRETION HUMAN - UNII:9BZG9E3I8F) GONORRHEAL URETHRAL SECRETION HUMAN 30 [hp_C] in 30 [hp_C] DATURA STRAMONIUM (UNII: G6W4F0V8Z3) (DATURA STRAMONIUM - UNII:G6W4F0V8Z3) DATURA STRAMONIUM 30 [hp_C] in 30 [hp_C] LYCOSA TARANTULA (UNII: 86M454L2TT) (LYCOSA TARANTULA - UNII:86M454L2TT) LYCOSA TARANTULA 30 [hp_C] in 30 [hp_C] VERATRUM ALBUM ROOT (UNII: QNS6W5US1Z) (VERATRUM ALBUM ROOT - UNII:QNS6W5US1Z) VERATRUM ALBUM ROOT 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0106-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 ABSENT MINDED
androc 30c, apis 30c, cannabis indica 30c, causticum 30c, chamomilla 30c, helleborus 30c, iridium met 30c, lachesis 30c, mezereum 30c, nat mur 30c, nux moschata 30c, platina 30c, pulsatilla 30c, sepia 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANDROCTONUS AUSTRALIS VENOM (UNII: 25IMQ489AW) (ANDROCTONUS AUSTRALIS VENOM - UNII:25IMQ489AW) ANDROCTONUS AUSTRALIS VENOM 30 [hp_C] in 30 [hp_C] APIS MELLIFERA VENOM (UNII: 76013O881M) (APIS MELLIFERA VENOM - UNII:76013O881M) APIS MELLIFERA VENOM 30 [hp_C] in 30 [hp_C] CANNABIS SATIVA SUBSP. INDICA TOP (UNII: FTS5RM302N) (CANNABIS SATIVA SUBSP. INDICA TOP - UNII:FTS5RM302N) CANNABIS SATIVA SUBSP. INDICA TOP 30 [hp_C] in 30 [hp_C] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] HELLEBORUS NIGER ROOT (UNII: 608DGJ6815) (HELLEBORUS NIGER ROOT - UNII:608DGJ6815) HELLEBORUS NIGER ROOT 30 [hp_C] in 30 [hp_C] IRIDIUM (UNII: 44448S9773) (IRIDIUM - UNII:44448S9773) IRIDIUM 30 [hp_C] in 30 [hp_C] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_C] in 30 [hp_C] DAPHNE MEZEREUM BARK (UNII: X2N6E405GV) (DAPHNE MEZEREUM BARK - UNII:X2N6E405GV) DAPHNE MEZEREUM BARK 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] NUTMEG (UNII: AEE24M3MQ9) (NUTMEG - UNII:AEE24M3MQ9) NUTMEG 30 [hp_C] in 30 [hp_C] PLATINUM (UNII: 49DFR088MY) (PLATINUM - UNII:49DFR088MY) PLATINUM 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0107-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 MEMORY BOOST
baryta carb 30c, carbolicum acidum 30c, calc carb 30c, calc phos 30c, chelidonium 30c, carboneum sulph 30c, hydrastis 30c, hyoscyamus 30c lac can 30c, nux moschata 30c, sulphur 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0108 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BARIUM CARBONATE (UNII: 6P669D8HQ8) (BARIUM CATION - UNII:V645272HLN) BARIUM CARBONATE 30 [hp_C] in 30 [hp_C] PHENOL (UNII: 339NCG44TV) (PHENOL - UNII:339NCG44TV) PHENOL 30 [hp_C] in 30 [hp_C] OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_C] in 30 [hp_C] TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 30 [hp_C] in 30 [hp_C] CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 30 [hp_C] in 30 [hp_C] CARBON DISULFIDE (UNII: S54S8B99E8) (CARBON DISULFIDE - UNII:S54S8B99E8) CARBON DISULFIDE 30 [hp_C] in 30 [hp_C] GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 30 [hp_C] in 30 [hp_C] HYOSCYAMUS NIGER (UNII: 4WRK2153H3) (HYOSCYAMUS NIGER - UNII:4WRK2153H3) HYOSCYAMUS NIGER 30 [hp_C] in 30 [hp_C] CANIS LUPUS FAMILIARIS MILK (UNII: G39P120JQT) (CANIS LUPUS FAMILIARIS MILK - UNII:G39P120JQT) CANIS LUPUS FAMILIARIS MILK 30 [hp_C] in 30 [hp_C] NUTMEG (UNII: AEE24M3MQ9) (NUTMEG - UNII:AEE24M3MQ9) NUTMEG 30 [hp_C] in 30 [hp_C] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0108-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 STYE IN THE EYE
calc fluor 30c,, conium mac 30c,, carboneum sulph 30c, digitalis 30c, graphites 30c, lycopodium 30c, pulsatilla 30c, sepia 30c, silicea 30c, staphysagria 30c, sulphur 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0109 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 30 [hp_C] in 30 [hp_C] CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 30 [hp_C] in 30 [hp_C] CARBON DISULFIDE (UNII: S54S8B99E8) (CARBON DISULFIDE - UNII:S54S8B99E8) CARBON DISULFIDE 30 [hp_C] in 30 [hp_C] DIGITALIS (UNII: F1T8QT9U8B) (DIGITALIS - UNII:F1T8QT9U8B) DIGITALIS 30 [hp_C] in 30 [hp_C] GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 30 [hp_C] in 30 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 30 [hp_C] DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0109-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 WEIGHT LOSS
ambra grisea 30c, antimonium crudum 30c, calc carb 30c, capsicum 30c, ferrum met 30c, graphites 30c, kali carb 30c, phytolacca 30c, sepia sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMBERGRIS (UNII: XTC0D02P6C) (AMBERGRIS - UNII:XTC0D02P6C) AMBERGRIS 30 [hp_C] in 30 [hp_C] ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 30 [hp_C] in 30 [hp_C] OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_C] in 30 [hp_C] CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSICUM 30 [hp_C] in 30 [hp_C] IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 30 [hp_C] in 30 [hp_C] GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 30 [hp_C] in 30 [hp_C] POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 30 [hp_C] in 30 [hp_C] PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0110-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 Labeler - Home Sweet Homeopathics (078883277) Establishment Name Address ID/FEI Business Operations Nichole Michelle Langham 078883277 manufacture(59667-0089, 59667-0090, 59667-0091, 59667-0092, 59667-0093, 59667-0094, 59667-0095, 59667-0096, 59667-0098, 59667-0099, 59667-0100, 59667-0101, 59667-0102, 59667-0103, 59667-0104, 59667-0105, 59667-0106, 59667-0107, 59667-0108, 59667-0109, 59667-0110) , label(59667-0089, 59667-0090, 59667-0091, 59667-0092, 59667-0093, 59667-0094, 59667-0095, 59667-0096, 59667-0098, 59667-0099, 59667-0100, 59667-0101, 59667-0102, 59667-0103, 59667-0104, 59667-0105, 59667-0106, 59667-0107, 59667-0108, 59667-0109, 59667-0110) , pack(59667-0089, 59667-0090, 59667-0091, 59667-0092, 59667-0093, 59667-0094, 59667-0095, 59667-0096, 59667-0098, 59667-0099, 59667-0100, 59667-0101, 59667-0102, 59667-0103, 59667-0104, 59667-0105, 59667-0106, 59667-0107, 59667-0108, 59667-0109, 59667-0110)