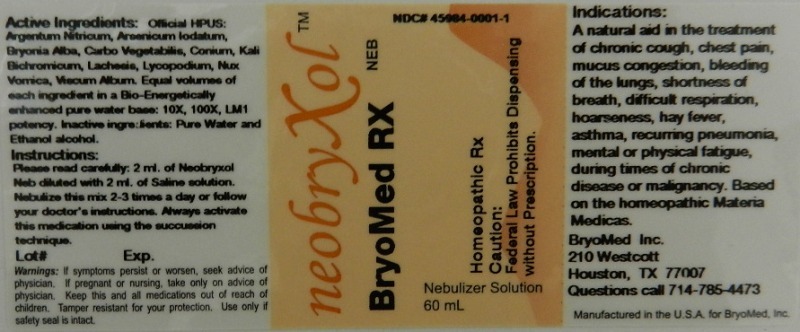
Label: NEOBRYXOL NEB- argentum nitricum, arsenicum iodatum, bryonia, carbo vegetabiles, conium maculatum, kali bichromicum, lachesis mutus, lycopodium, nux vomica and viscum album liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 45984-0001-1 - Packager: Bryomed Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 21, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Inactive Ingredients
- Dosage and Administration
-
Purpose
A natural aid in the treatment of:
- chronic cough
- chest pain
- mucus congestion
- bleeding of the lungs
- shortness of breath
- difficult respiration
- hoarseness
- hay fever
- asthma
- recurring pneumonia
- mental or physical fatigue during times of chronic disease or malignancy.
Reference image neb.jpg
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Indications and Usage
A natural aid in the treatment of chronic cough, chest pain, mucus congestion, bleeding of the lungs, shortness of breath, difficult respiration, hoarseness, hay fever, asthma, recurring pneumonia, mental or physical fatigue, during times of chronic disease or malignancy. Based on the homeopathic Materia Medicas.
Reference image neb.jpg - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEOBRYXOL NEB
argentum nitricum, arsenicum iodatum, bryonia, carbo vegetabiles, conium maculatum, kali bichromicum, lachesis mutus, lycopodium, nux vomica and viscum album liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:45984-0001 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 60 mL ARSENIC TRIIODIDE (UNII: 3029988O2T) (ARSENIC TRIIODIDE - UNII:3029988O2T) ARSENIC TRIIODIDE 10 [hp_X] in 60 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 60 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 10 [hp_X] in 60 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 10 [hp_X] in 60 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM DICHROMATE 10 [hp_X] in 60 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 10 [hp_X] in 60 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 10 [hp_X] in 60 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 10 [hp_X] in 60 mL VISCUM ALBUM WHOLE (UNII: E6839Q6DO1) (VISCUM ALBUM WHOLE - UNII:E6839Q6DO1) VISCUM ALBUM WHOLE 10 [hp_X] in 60 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45984-0001-1 60 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/04/2012 Labeler - Bryomed Inc (078382220) Registrant - Bryomed Inc (078382220)