Label: NYSTATIN CREAM cream
- NDC Code(s): 0316-0221-15, 0316-0221-30
- Packager: Crown Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx Only
-
DESCRIPTION
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces nursei.
Structural formula:
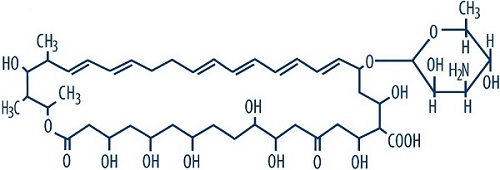
C 47H 75NO 17 Molecular Weight: 926.13
Nystatin cream is for dermatologic use.
Each gram contains 100,000 USP Nystatin Units in an aqueous, cream base consisting of purified water, emulsifying wax, mineral oil, propylene glycol, sorbitol solution, cetyl palmitate, sorbic acid and potassium sorbate.
- CLINICAL PHARMACOLOGY
-
Microbiology
Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsis glabrata, Tricophyton rubrum, T. mentagrophytes. Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing levels of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida (C. tropicalis, C. guilliermondi, C. krusei, and C. stellatoides) become quite resistant on treatment with nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Nystatin cream should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens.
-
INFORMATION FOR THE PATIENT
Patients using this medication should receive the following information and instructions:
1. The patient should be instructed to use this medication as directed (including the replacement of missed doses). This medication is not for any disorder other than that for which it is prescribed.
2. Even if symptomatic relief occurs within the first few days of treatment, the patient should be advised not to interrupt or discontinue therapy until the prescribed course of treatment is completed.
3. If symptoms of irritation develop, the patient should be advised to notify the physician promptly. - Laboratory Tests
- Carcinogenesis, Mutagenesis, Impairment of Fertility
-
Pregnancy
Teratogenic Effects
Category C
Animal reproduction studies have not been conducted with any nystatin cream. It also is not known whether this cream can cause fetal harm when used by a pregnant woman or can affect reproductive capacity. Nystatin cream should be prescribed for a pregnant woman only if the potential benefit to the mother outweighs the potential risk to the fetus. - Nursing Mothers
- Pediatric Use
-
Geriatric Use
Clinical studies with nystatin cream did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
ADVERSE REACTIONS
The frequency of adverse events reported in patients using nystatin cream is less than 0.1%. The more common events that were reported include allergic reactions, burning, itching, rash, eczema, and pain on application. (See PRECAUTIONS: General.)
To report SUSPECTED ADVERSE REACTIONS, contact Crown Laboratories, Inc. at 1-423-926-4413 or FDA at 1-800-FDA-1088 or https://www.fda.gov/Safety/MedWatch/
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Nystatin Cream USP is a light yellow to yellow cream that is supplied in:
15 gram tube NDC 0316-0221-15
30 gram tube NDC 0316-0221-30
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Avoid freezing.
Manufactured and Distributed by:
Crown Laboratories, Inc., Johnson City, TN 37604Printed in USA
P9526.00
-
15 gram tube
NDC 0316-0221-15
Rx Only
Nystatin Cream, USP
100,000 units per gram
WARNING: Keep out of reach of children.
For external use only.
Not for ophthalmic use.
15 grams
Each gram contains: 100,000 USP Nystatin Units in an aqueous, cream base consisting of purified water, emulsifying wax, mineral oil, propylene glycol, sorbitol solution, cetyl palmitate, sorbic acid and potassium sorbate.
TO OPEN: Use cap to puncture seal.
IMPORTANT: Do not use if seal has been punctured or is not visible.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Avoid freezing.
Usual Dosage: Apply liberally to affected area twice daily. See package insert for full prescribing information.
See crimp of tube for Lot Number and Expiration Date.
Manufactured and Distributed by:
Crown Laboratories, Inc., Johnson City, TN 37604
P9503.01

-
15 gram carton
NDC 0316-0221-15
Rx Only
Nystatin Cream, USP
100,000 units per gram
WARNING:Keep out of reach of children.
For external use only.
Not for ophthalmic use.
15 grams
Each gram contains: 100,000 USP Nystatin Units in an aqueous, cream base consisting of purified water, emulsifying wax, mineral oil, propylene glycol, sorbitol solution, cetyl palmitate, sorbic acid and potassium sorbate.
Directions for puncturing tube seal: Remove cap. Turn cap upside down and place puncture tip onto tube seal. Push cap down until seal is punctured. Screw cap back on to close.
IMPORTANT: Do not use if seal has been punctured or is not visible.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Avoid freezing.
Usual Dosage: Apply liberally to affected area twice daily. See package insert for full prescribing information.
See end of carton for Lot Number and Expiration Date.
Manufactured and Distributed by:
Crown Laboratories, Inc., Johnson City, TN 37604
P9515.00

-
INGREDIENTS AND APPEARANCE
NYSTATIN CREAM
nystatin cream creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0316-0221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NYSTATIN (UNII: BDF1O1C72E) (NYSTATIN - UNII:BDF1O1C72E) NYSTATIN 100000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) SORBIC ACID (UNII: X045WJ989B) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) CETYL PALMITATE (UNII: 5ZA2S6B08X) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0316-0221-15 1 in 1 CARTON 10/10/2017 1 15 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0316-0221-30 1 in 1 CARTON 10/10/2017 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207733 10/10/2017 Labeler - Crown Laboratories (079035945) Establishment Name Address ID/FEI Business Operations Crown Laboratories 079035945 manufacture(0316-0221)


