Label: WORKFORCE PERSONAL CARE HAND SANITIZER UNSCENTED- ethyl alcohol hand sanitizer gel
- NDC Code(s): 71824-7700-1, 71824-7702-1, 71824-7708-1, 71824-7716-1
- Packager: Prima Fleur Botanicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
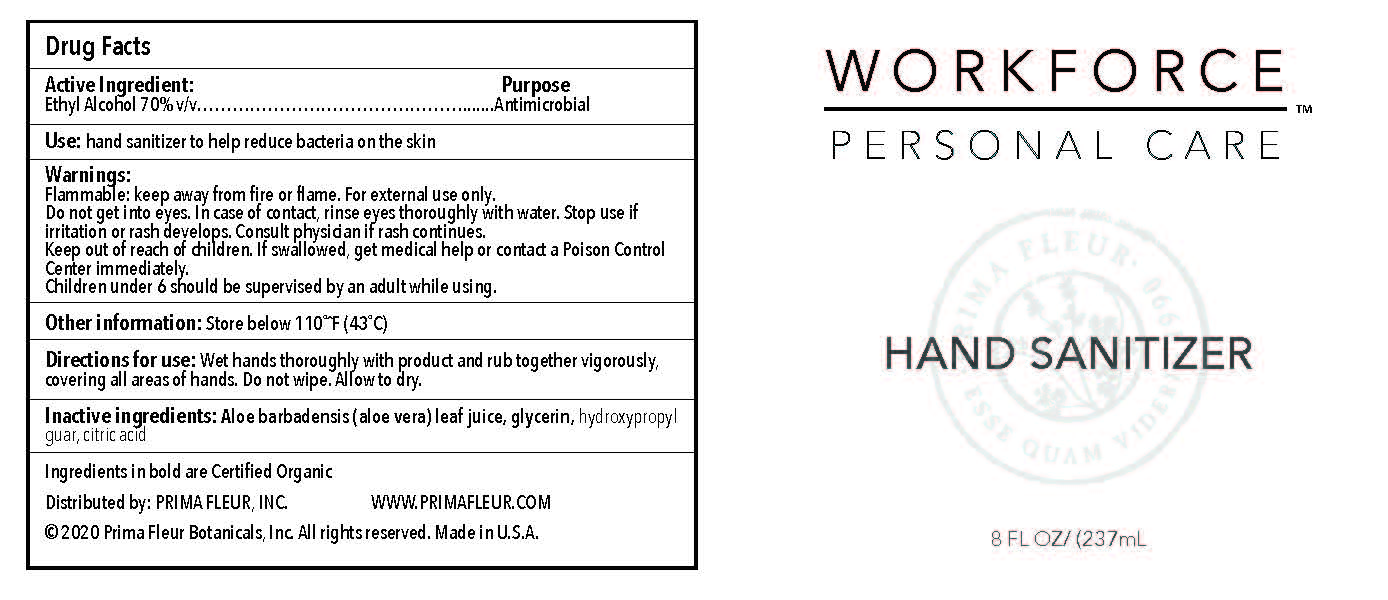
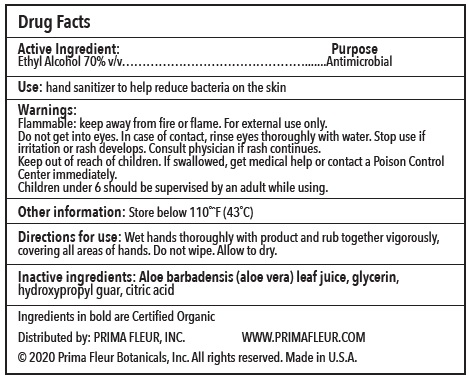
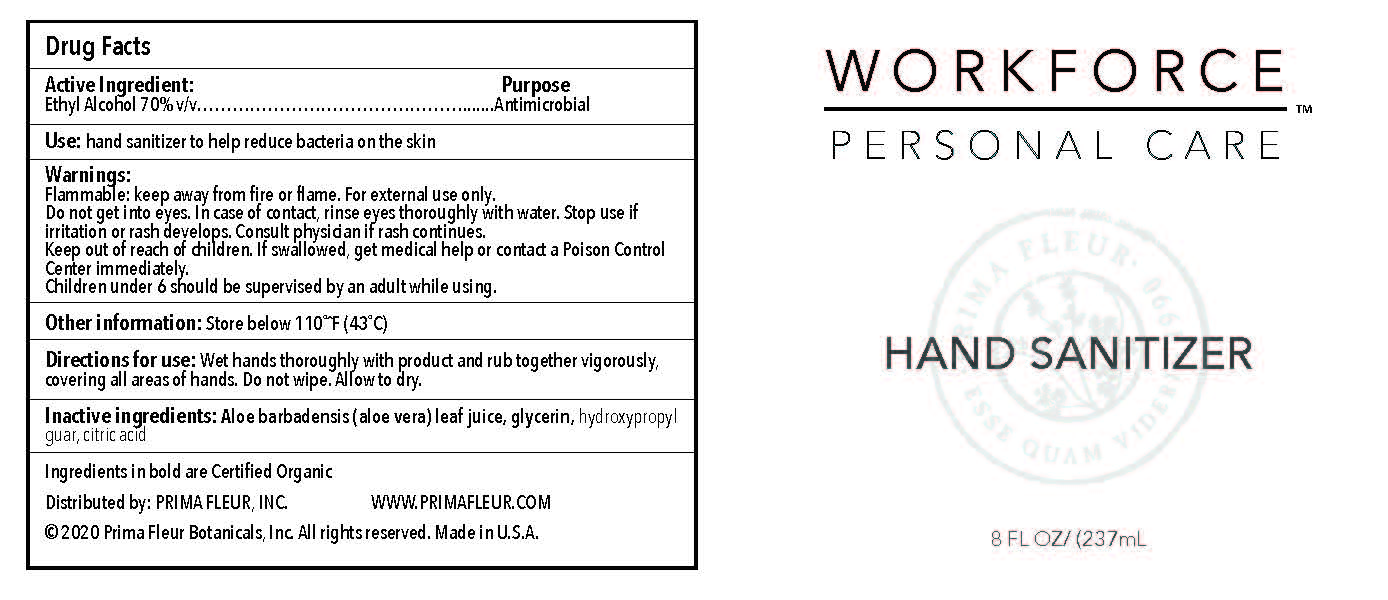
- Directions for Use
- Warnings
- Inactive Ingredients
- Use
- Warnings
- Use
- Active ingredients
- Workforce Personal Care Hand Sanitizer Unscented
- 8oz label
- 16 oz front label
- 128oz (gallon) front label
-
INGREDIENTS AND APPEARANCE
WORKFORCE PERSONAL CARE HAND SANITIZER UNSCENTED
ethyl alcohol hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71824-7708 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 165.6116 mL in 236.588 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color white (Clear gel) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71824-7708-1 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/28/2020 WORKFORCE PERSONAL CARE HAND SANITIZER UNSCENTED
ethyl alcohol hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71824-7700 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 2649.787 mL in 3785.41 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color white (Clear gel) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71824-7700-1 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/28/2020 WORKFORCE PERSONAL CARE HAND SANITIZER UNSCENTED
ethyl alcohol hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71824-7702 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 41.40297 mL in 59.1471 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color white (Clear gel) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71824-7702-1 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/21/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/08/2020 WORKFORCE PERSONAL CARE HAND SANITIZER UNSCENTED
ethyl alcohol hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71824-7716 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 331.2232 mL in 473.176 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color white (Clear gel) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71824-7716-1 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/28/2020 Labeler - Prima Fleur Botanicals, Inc. (825058308)