Label: KLARON- sulfacetamide sodium lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5401-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0066-7500
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 2, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each mL of Klaron® (sodium sulfacetamide lotion) Lotion, 10% contains 100 mg of sodium sulfacetamide in a vehicle consisting of purified water; propylene glycol; lauramide DEA (and) diethanolamine; polyethylene glycol 400, monolaurate; hydroxyethyl cellulose; sodium chloride; sodium metabisulfite; methylparaben; xanthan gum; EDTA and simethicone.
Sodium sulfacetamide is a sulfonamide with antibacterial activity. Chemically, sodium sulfacetamide is N'-[(4-aminophenyl)sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
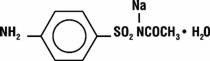
-
CLINICAL PHARMACOLOGY
The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory, based on sulfonamides acting as a competitive inhibitor of para-aminobenzoic acid (PABA) utilization, an essential component for bacterial growth. While absorption through intact skin in humans has not been determined, in vitro studies with human cadaver skin indicated a percutaneous absorption of about 4%. Sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine largely unchanged. The biological half-life has been reported to be between 7 to 13 hours.
The pharmacokinetics of sulfacetamide and its major metabolite sulfaniliamide in Klaron Lotion was evaluated in adult subjects (N=14) with acne vulgaris. The subjects applied Klaron Lotion to their face, back, chest and shoulders every 12 hours for 28 days. The percentage of the applied dose of Klaron Lotion excreted in the urine as sulfacetamide plus sulfanilamide, ranged from 0.08 to 0.33%.
- INDICATIONS
-
CONTRAINDICATIONS
Klaron Lotion is contraindicated for use by patients having known hypersensitivity to sulfonamides or any other component of this preparation (see WARNINGS section).
-
WARNINGS
Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias. Hypersensitivity reactions may occur when a sulfonamide is readministered, irrespective of the route of administration. Sensitivity reactions have been reported in individuals with no prior history of sulfonamide hypersensitivity. At the first sign of hypersensitivity, skin rash or other reactions, discontinue use of this preparation (see ADVERSE REACTIONS section).
Klaron Lotion contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people (see CONTRAINDICATIONS section).
-
PRECAUTIONS
General
For external use only. Keep away from eyes. If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. Hypersensitivity reactions may occur when a sulfonamide is readministered irrespective of the route of administration, and cross-sensitivity between different sulfonamides may occur. Sodium sulfacetamide can cause reddening and scaling of the skin. Particular caution should be employed if areas of involved skin to be treated are denuded or abraded.
Keep out of the reach of children.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pregnancy – Category C
Animal reproduction studies have not been conducted with Klaron® Lotion. It is also not known whether Klaron Lotion can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Klaron Lotion should be given to a pregnant woman only if clearly needed.
Kernicterus may occur in the newborn as a result of treatment of a pregnant woman at term with orally administered sulfonamide. There are no adequate and well controlled studies of Klaron Lotion in pregnant women, and it is not known whether topically applied sulfonamides can cause fetal harm when administered to a pregnant woman.
Nursing Mothers
It is not known whether sodium sulfacetamide is excreted in the human milk following topical use of Klaron Lotion. Systemically administered sulfonamides are capable of producing kernicterus in the infants of lactating women. Small amounts of orally administered sulfonamides have been reported to be eliminated in human milk. Because many drugs are excreted in human milk, caution should be exercised in prescribing for nursing women.
-
ADVERSE REACTIONS
In controlled clinical trials for the management of acne vulgaris, the occurrence of adverse reactions associated with the use of Klaron Lotion was infrequent and restricted to local events. The total incidence of adverse reactions reported in these studies was less than 2%. Only one of 105 patients treated with Klaron Lotion had adverse reactions of erythema, itching and edema. It has been reported that sodium sulfacetamide may cause local irritation, stinging and burning. While the irritation may be transient, occasionally, the use of medication has to be discontinued.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 118mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
KLARON
sulfacetamide sodium lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5401(NDC:0066-7500) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sulfacetamide sodium (UNII: 4NRT660KJQ) (sulfacetamide - UNII:4965G3J0F5) sulfacetamide sodium 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) propylene glycol (UNII: 6DC9Q167V3) Lauric Diethanolamide (UNII: I29I2VHG38) diethanolamine (UNII: AZE05TDV2V) polyethylene glycol 400 (UNII: B697894SGQ) sodium chloride (UNII: 451W47IQ8X) sodium metabisulfite (UNII: 4VON5FNS3C) methylparaben (UNII: A2I8C7HI9T) xanthan gum (UNII: TTV12P4NEE) EDETIC ACID (UNII: 9G34HU7RV0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5401-0 118 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019931 09/01/2005 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel


