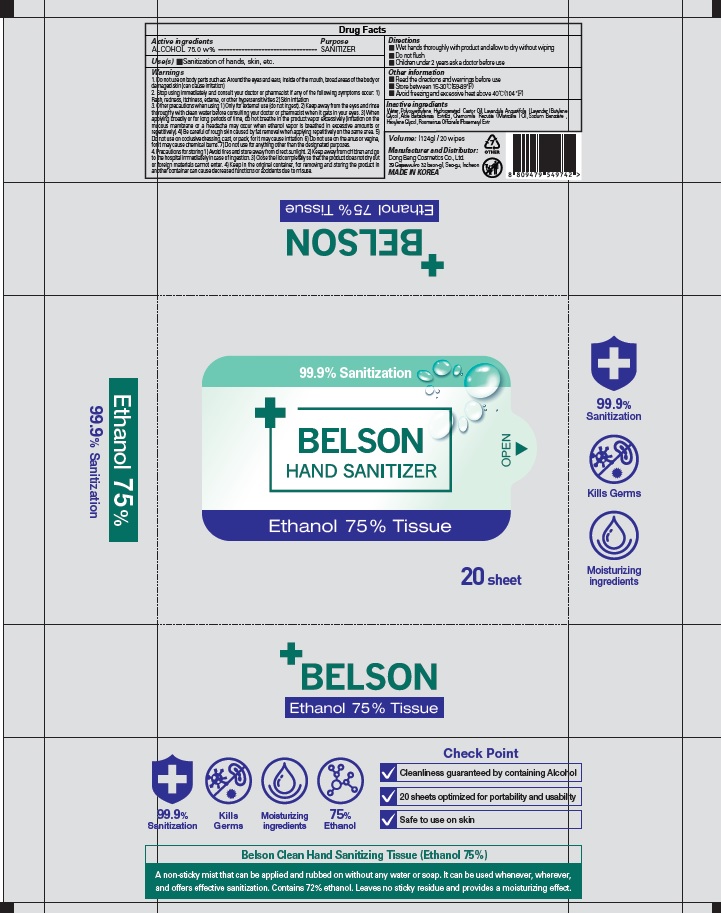
Label: BELSON HAND SANITIZER ETHANOL 75 PERCENT TISSUE- alcohol liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 74002-3040-1 - Packager: DONGBANGCOSMETICS CO.,LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 15, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
1. Do not use on body parts such as: Around the eyes and ears, inside of the mouth, broad areas of the body or damaged skin (can cause irritation)
2. Stop using immediately and consult your doctor or pharmacist if any of the following symptoms occur: 1) Rash, redness, itchiness, edema, or other hypersensitivities 2) Skin irritation
3. Other precautions when using 1) Only for external use (do not ingest). 2) Keep away from the eyes and rinse thoroughly with clean water before consulting your doctor or pharmacist when it gets in your eyes. 3) When applying broadly or for long periods of time, do not breathe in the product vapor excessively (irritation on the mucous membrane or a headache may occur when ethanol vapor is breathed in excessive amounts or repetitively). 4) Be careful of rough skin caused by fat removal when applying repetitively on the same area. 5) Do not use on occlusive dressing, cast, or pack, for it may cause irritation. 6) Do not use on the anus or vagina, for it may cause chemical burns. 7) Do not use for anything other than the designated purposes.
4. Precautions for storing 1) Avoid fires and store away from direct sunlight. 2) Keep away from children and go to the hospital immediately in case of ingestion. 3) Close the lid completely so that the product does not dry out or foreign materials cannot enter. 4) Keep in the original container, for removing and storing the product in another container can cause decreased functions or accidents due to misuse. - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Other lnformation
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BELSON HAND SANITIZER ETHANOL 75 PERCENT TISSUE
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74002-3040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 75 g in 100 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) PEG-60 Hydrogenated Castor Oil (UNII: 02NG325BQG) LAVENDER OIL (UNII: ZBP1YXW0H8) Butylene Glycol (UNII: 3XUS85K0RA) ALOE VERA LEAF (UNII: ZY81Z83H0X) Sodium Benzoate (UNII: OJ245FE5EU) Hexylene Glycol (UNII: KEH0A3F75J) ROSEMARY (UNII: IJ67X351P9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74002-3040-1 124 g in 1 CONTAINER; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 06/01/2020 Labeler - DONGBANGCOSMETICS CO.,LTD (694452564) Registrant - DONGBANGCOSMETICS CO.,LTD (694452564) Establishment Name Address ID/FEI Business Operations DONGBANGCOSMETICS CO.,LTD 694452564 manufacture(74002-3040)