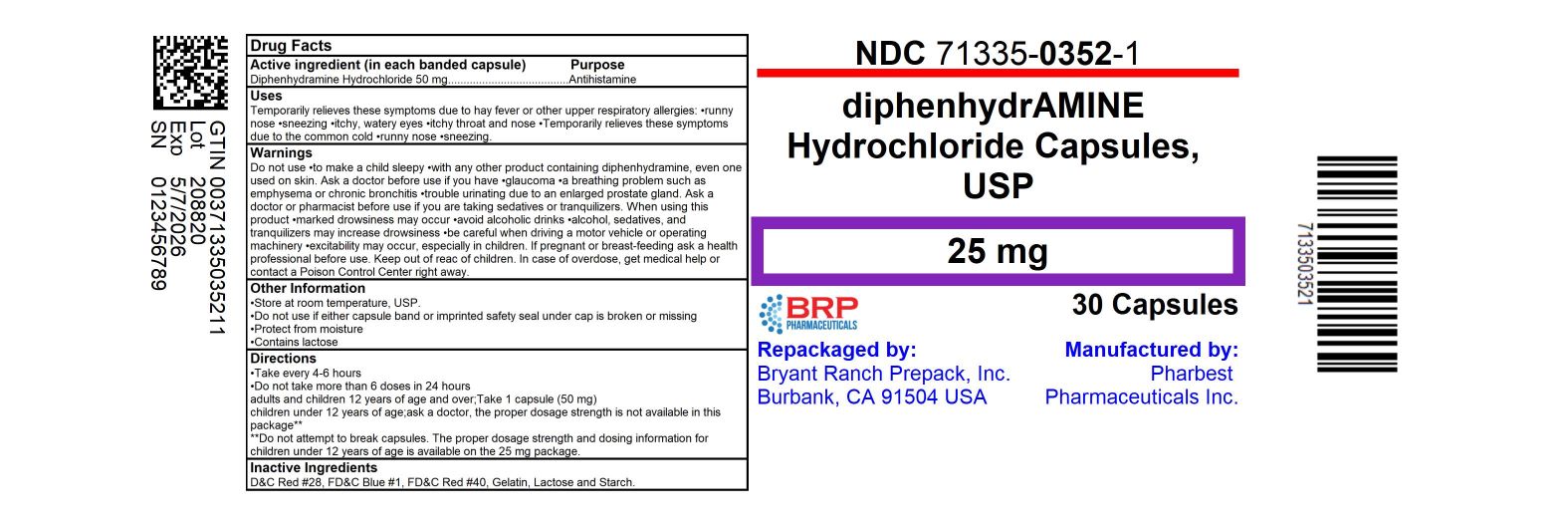
Label: DIPHENHYDRAMINE HCL capsule
-
NDC Code(s):
71335-0352-0,
71335-0352-1,
71335-0352-2,
71335-0352-3, view more71335-0352-4, 71335-0352-5, 71335-0352-6, 71335-0352-7, 71335-0352-8, 71335-0352-9
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 66424-020
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each capsule)
- Uses:
- Warnings:
- Do not use
- Ask a doctor or pharmacist before use
- When using this product
- If pregnant or breast-feeding,
- Keep out of reach of children
- Directions:
- Other information:
- Inactive ingredients: Black Iron Oxide, D & C Red #28, FD & C Blue #1, FD & C Red #40, Gelatin, Lactose Monohydrate, Magnesium Stearate, Silicon Dioxide, Sodium Lauryl Sulfate
-
HOW SUPPLIED
Diphenhydramine HCl 25 mg
- NDC: 71335-0352-1: 30 Capsules in a BOTTLE
- NDC: 71335-0352-2: 20 Capsules in a BOTTLE
- NDC: 71335-0352-3: 42 Capsules in a BOTTLE
- NDC: 71335-0352-4: 24 Capsules in a BOTTLE
- NDC: 71335-0352-5: 15 Capsules in a BOTTLE
- NDC: 71335-0352-6: 60 Capsules in a BOTTLE
- NDC: 71335-0352-7: 10 Capsules in a BOTTLE
- NDC: 71335-0352-8: 6 Capsules in a BOTTLE
- NDC: 71335-0352-9: 90 Capsules in a BOTTLE
- NDC: 71335-0352-0: 100 Capsules in a BOTTLE
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HCL
diphenhydramine hcl capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71335-0352(NDC:66424-020) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color pink Score no score Shape CAPSULE Size 14mm Flavor Imprint Code PH014 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-0352-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/19/2018 2 NDC:71335-0352-2 20 in 1 BOTTLE; Type 0: Not a Combination Product 11/21/2018 3 NDC:71335-0352-3 42 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 4 NDC:71335-0352-4 24 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 5 NDC:71335-0352-5 15 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 6 NDC:71335-0352-6 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 7 NDC:71335-0352-7 10 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 8 NDC:71335-0352-8 6 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 9 NDC:71335-0352-9 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 10 NDC:71335-0352-0 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/27/2010 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-0352) , RELABEL(71335-0352)