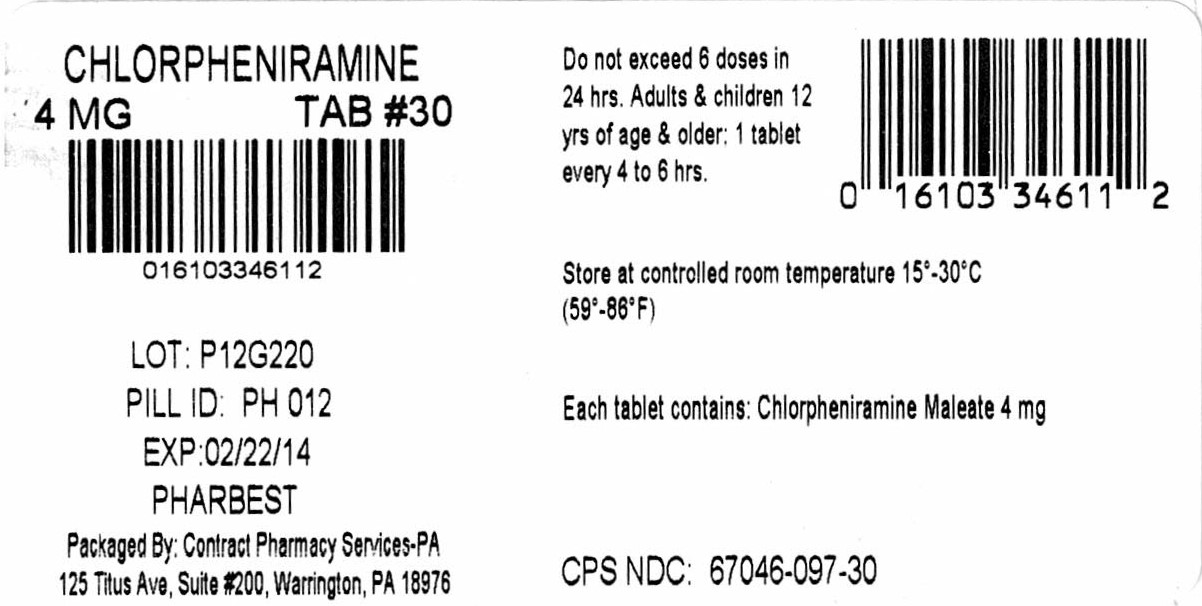
Label: CHLORPHENIRAMINE MALEATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 67046-097-07, 67046-097-14, 67046-097-15, 67046-097-21, view more67046-097-28, 67046-097-30, 67046-097-60 - Packager: Contract Pharmacy Services-PA
- This is a repackaged label.
- Source NDC Code(s): 66424-034
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 19, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(in each tablet)
- Uses
- Warnings
- Ask a doctor before use if you have,
- Ask a doctor or pharmacist before use if you
- When using this product
- If pregnant or breast feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients: corn starch, croscarmellose sodium, D&C yellow # 10 aluminum lake 14-18%, dicalcium phosphate, magnesium stearate, microcrystalline cellulose, silicon dioxide
- Questions:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHLORPHENIRAMINE MALEATE
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67046-097(NDC:66424-034) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color yellow Score no score Shape ROUND Size 8mm Flavor Imprint Code PH012 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67046-097-14 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/19/2017 2 NDC:67046-097-07 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/19/2017 3 NDC:67046-097-15 15 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/19/2017 4 NDC:67046-097-21 21 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/19/2017 5 NDC:67046-097-28 28 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/19/2017 6 NDC:67046-097-30 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/19/2017 7 NDC:67046-097-60 60 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/19/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 09/19/2017 Labeler - Contract Pharmacy Services-PA (945429777) Establishment Name Address ID/FEI Business Operations Coupler Enterprises 945429777 repack(67046-097)