Label: GAINPRO 10- bambermycins type a medicated article powder
- NDC Code(s): 23243-2070-5
- Packager: Huvepharma, Inc.
- Category: OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated May 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- RESIDUE WARNING
-
ADVERSE REACTIONS
To report suspected adverse drug events, for technical assistance or to obtain a copy
of the Safety Data Sheet (SDS), contact Huvepharma, Inc. at 1-877-994-4883 or
www.huvepharma.us. For additional information about adverse drug experience
reporting for animal drugs, contact FDA at 1-888-FDA-VETS or
http://www.fda.gov/reportanimalae. -
VETERINARY INDICATIONS
INDICATIONS FOR USE:
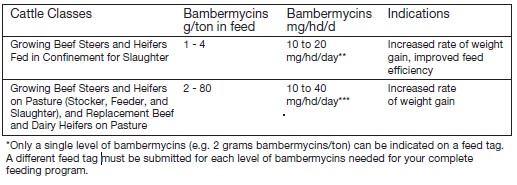
For increased rate of weight gain and improved feed efficiency in growing beef steers and heifers
fed in confinement for slaughter. For increased rate of weight gain in growing beef steers and
heifers on pasture (stocker, feeder, and slaughter), and replacement beef and dairy heifers on pasture. - ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- VETERINARY INDICATIONS
- USER SAFETY WARNINGS
- STORAGE AND HANDLING
- INDICATIONS FOR USE
-
INDICATIONS & USAGE
GROWING BEEF STEERS AND HEIFERS FED IN CONFINEMENT FOR SLAUGHTER:
**THE APPROVED DOSE IN GROWING BEEF STEERS AND HEIFERS FED IN CONFINEMENT
FOR SLAUGHTER IS 10 TO 20 MG OF BAMBERMYCINS/HD/DAY. The amount of bambermycins
will range between 1 and 4 grams per ton of feed depending upon the daily feed consumption
of the cattle. The amount of Type A Medicated Article added to the complete ration (Type C
Medicated Feed) must be based on daily feed consumption, the number of animals in the pen
and the level (10-20 mg/hd/day) of bambermycins being fed. From this information the grams
of bambermycins per ton of feed can be calculated in order to fulfill feed assay requirements.
See the following table for conversions from mg/hd/day to g/t.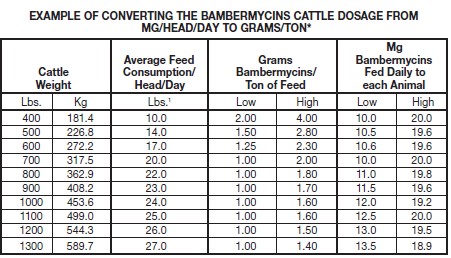
*Dosage range is 10 to 20 mg/head/day
1Assumes as fed ad libitum consumption and average daily gain of 2.0 to 3.0 lbs./dayExample Calculation—Amount of GAINPRO®-10 added to feed for a pen of cattle.
Given: Average Weight—800 lbs.; Number in pen—100 hd.; Dosage—20 mg/hd/day; Daily
Feed Consumption—22 lbs./day.
• 100 hd x 20 mg = 2,000 mg or 2 grams bambermycins.
• GAINPRO®-10 contains 10 grams bambermycins/lb., therefore add 0.2 pound of
GAINPRO®-10 to the daily feed for this pen of 100 animals.
• 22 lbs./day x 100 hd = 2,200 lbs. total feed consumption.
• 2 grams bambermycins ÷ 2,200 lbs. = .000909 g/lb.
• .000909 x 2,000 lbs. = 1.82 g bambermycins/ton of feed. -
INDICATIONS & USAGE
MIXING DIRECTIONS:
It is recommended that GAINPRO®-10 be diluted with a feed ingredient before addition
to the final feed. A dilution of 1 part of GAINPRO®-10 and 9 parts feed ingredient is the
suggested working intermediate pre-blend. The table below shows examples of
GAINPRO®-10 and working intermediate pre-blend addition levels for various commonly
used feeds.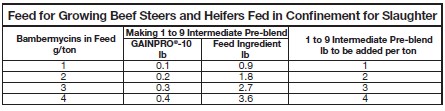
Thoroughly mix both working intermediate pre-blend and finished feed to ensure complete
and uniform distribution of the GAINPRO®-10.FEEDING DIRECTIONS:
Feed continuously in a complete feed at the rate of 10-20 mg bambermycins/head/day from
the Type C Medicated Feed containing 1-4 g bambermycins/ton.TYPE B LIQUID MEDICATED FEED INTENDED FOR ADDITION TO DRY FEEDS FOR
GROWING BEEF STEERS AND HEIFERS FED IN CONFINEMENT FOR SLAUGHTER:
•Type B Liquid Medicated Feed should be in a pH range of 3.8 to 7.5 and should have a moisture
content between 30 and 45%.
•Type B Liquid Medicated Feed must be mixed into the dry feed within 8 weeks of manufacture.
•Type B Liquid Medicated Feed can be manufactured containing 40.0 to 800.0 g of bambermycins/
ton. Mixing directions into dry feed are as follows:
Before feeding, the Type B Liquid Medicated Feed must be thoroughly mixed with other feed
materials to make a complete feed (Type C Medicated Feed). Examples of addition rates to
complete feeds are shown in the following table.Bambermycins (g/ton) in
Type B Liquid
Medicated FeedAddition Rate of Type B
Liquid Medicated Feed
(lb/ton) to Complete FeedBambermycins (g/ton) in
Final Complete Feed
(Type C Medicated Feed)40.0
50.0
1.0
40.0
200.0
4.0
100.0
20.0
1.0
100.0
80.0
4.0
400.0
5.0
1.0
400.0
20.0
4.0
800.0
2.5
1.0
800.0
10.0
4.0
The complete dry feed (Type C Medicated Feed) may only be fed to growing beef steers and heifers
fed in confinement for slaughter and must be fed within one week after mixing.GROWING BEEF STEERS AND HEIFERS ON PASTURE (STOCKER, FEEDER, AND SLAUGHTER),
AND REPLACEMENT BEEF AND DAIRY HEIFERS ON PASTURE:
***THE APPROVED DOSE IN GROWING BEEF STEERS AND HEIFERS ON PASTURE (STOCKER,
FEEDER, AND SLAUGHTER), AND REPLACEMENT BEEF AND DAIRY HEIFERS ON PASTURE IS
10 TO 40 MG OF BAMBERMYCINS/HD/DAY. Daily intakes of bambermycins in excess of
20 mg/head/day have not been shown to be more effective than 20 mg/head/day.MIXING DIRECTIONS:
It is recommended that GAINPRO®-10 be diluted with a feed ingredient before addition to the
supplemental feed. A dilution of 1 part of GAINPRO®-10 and 9 parts feed ingredient is the suggested
working intermediate pre-blend. The table below shows examples of GAINPRO®-10
and working intermediate pre-blend addition levels and feeding rates.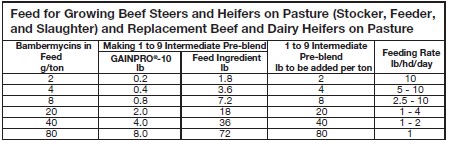
Thoroughly mix both working intermediate pre-blend and finished feed to ensure complete and
uniform distribution of the GAINPRO®-10.To manufacture Type B medicated feeds containing 100.0 to 800.0 g/ton bambermycins
mix 10 to 80 lbs. of GAINPRO®-10 with 1990 to 1920 lbs. of non-medicated feed.To manufacture Type C medicated feeds containing 2.0 to 80.0 g/ton bambermycins from
Type B medicated feeds mix:
40 to 1600 lbs. of Type B feed containing 100 g/ton bambermycins with 1960 to 400 lbs.
of non-medicated feed, or
5 to 200 lbs. of Type B feed containing 800 g/ton bambermycins with 1995 to 1800
lbs. of non-medicated feed.
Thoroughly mix both Type B medicated feed and finished feed to ensure complete and
uniform distribution of the GAINPRO®-10.FEEDING DIRECTIONS FOR HAND-FED TYPE C MEDICATED FEED:
Feed continuously on a hand-fed basis at a rate of 10 to 40 mg bambermycins per head
per day. The drug must be contained in 1 to 10 lbs. of supplemental Type C medicated
feed containing 2 to 80 g bambermycins per ton. Daily intakes of bambermycins in
excess of 20 mg/head/day have not been shown to be more effective than 20 mg/head/day.TYPE C FREE-CHOICE MEDICATED FEED:
An FDA approved formula must be used to manufacture free-choice medicated feeds containing
bambermycins. Feed mills that use a proprietary formula are required to be licensed.HUVEPHARMA®
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GAINPRO 10
bambermycins type a medicated article powderProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:23243-2070 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BAMBERMYCINS (UNII: PP922A42V2) (BAMBERMYCINS - UNII:PP922A42V2) BAMBERMYCINS 22 g in 1 kg Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) ETHOXYQUIN (UNII: 9T1410R4OR) Product Characteristics Color brown ((Off White to Tan)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-2070-5 22.68 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141034 08/22/2016 Labeler - Huvepharma, Inc. (619153559) Registrant - Huvepharma EOOD (552671651) Establishment Name Address ID/FEI Business Operations Huvepharma, Inc. 883128204 manufacture, analysis, label, pack, medicated animal feed manufacture