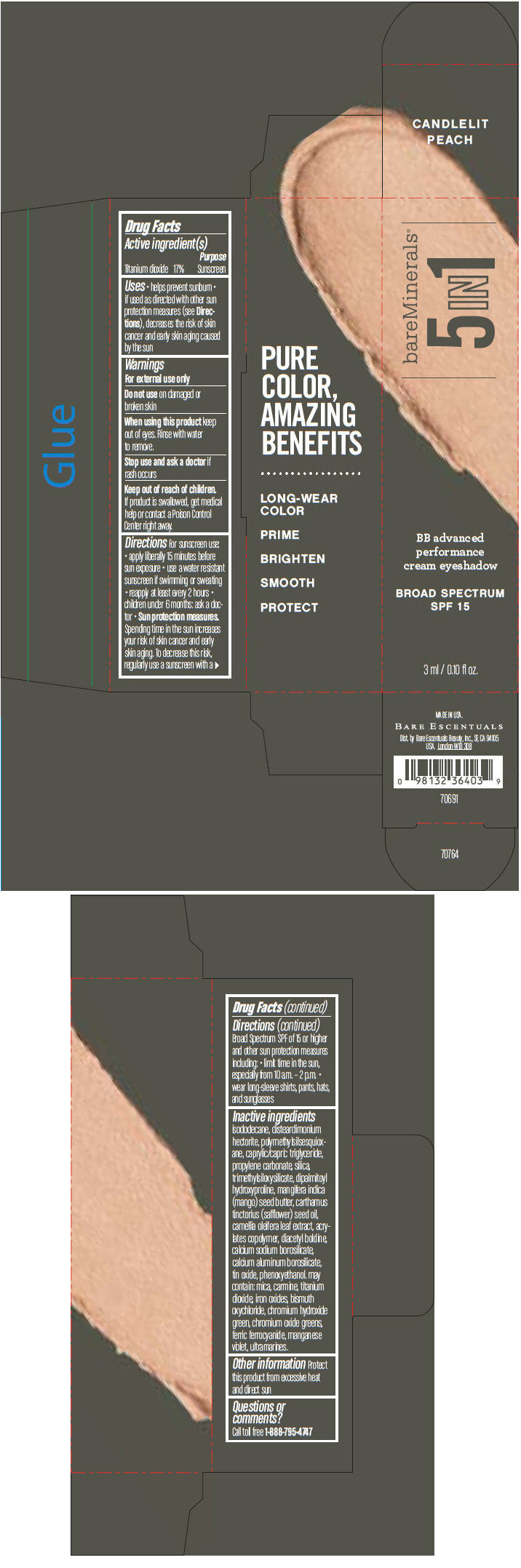
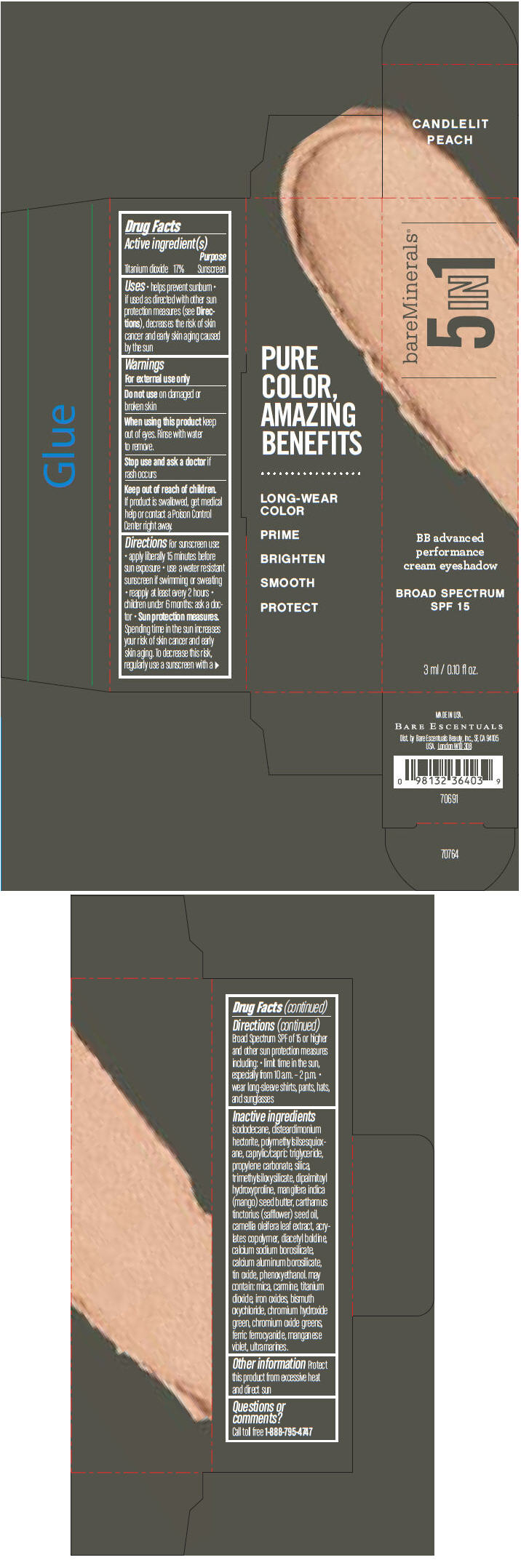
Label: BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 CANDLELIT PEACH- titanium dioxide cream
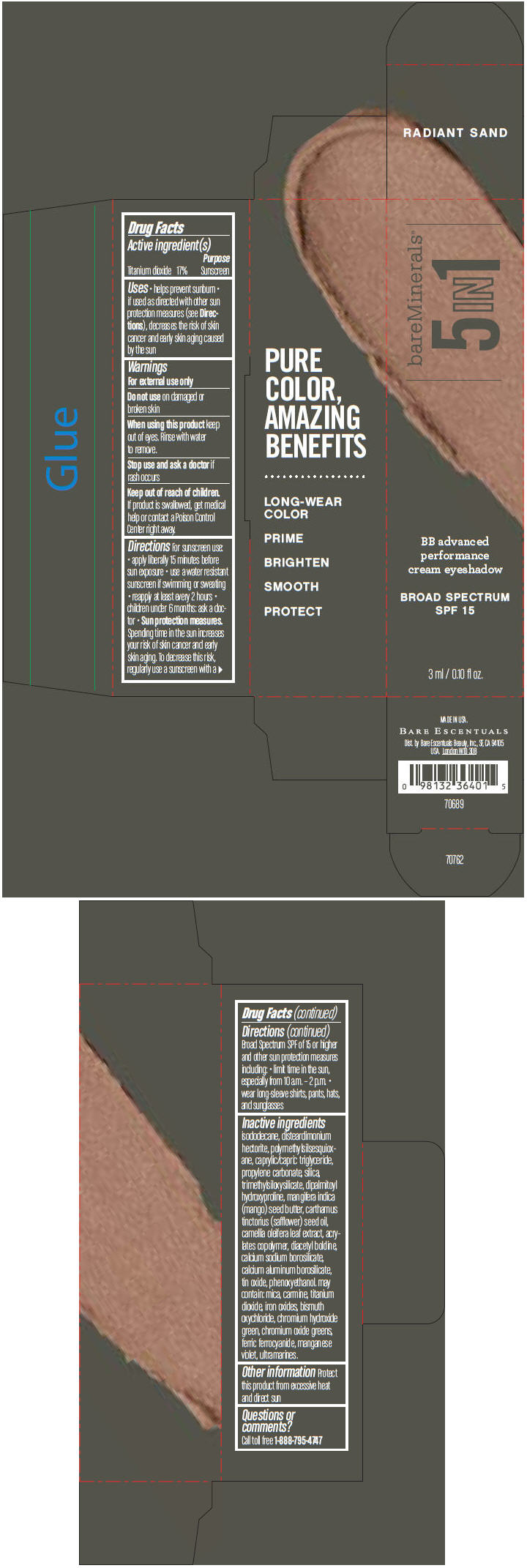
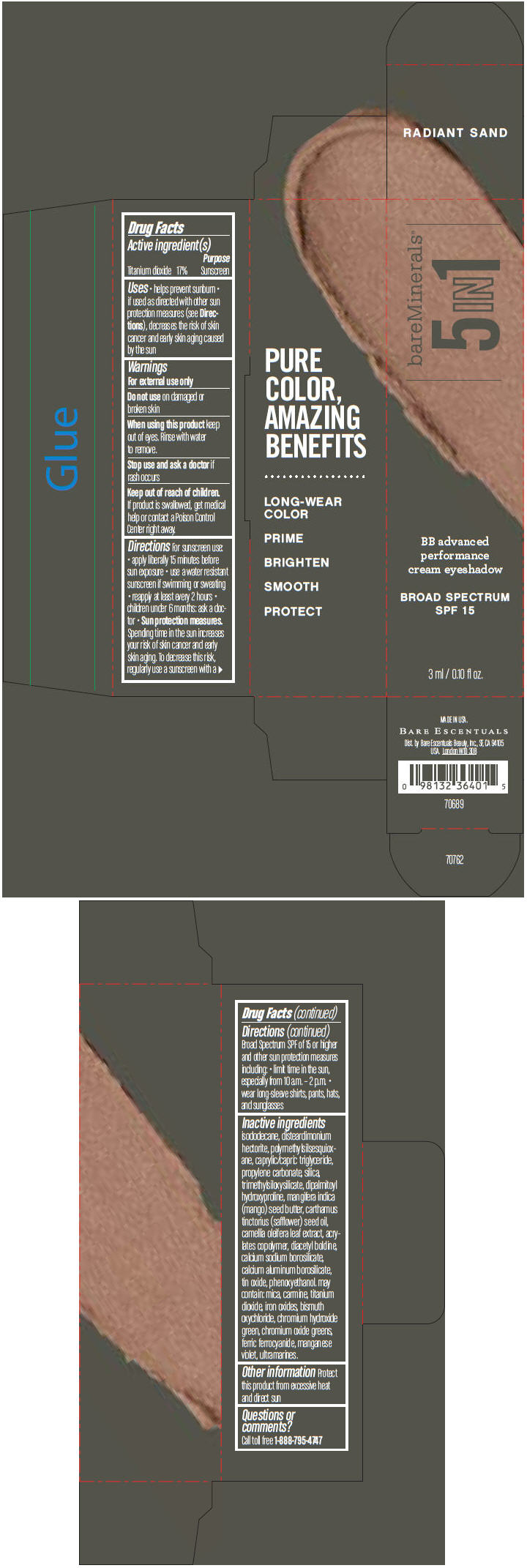
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 RADIANT SAND- titanium dioxide cream
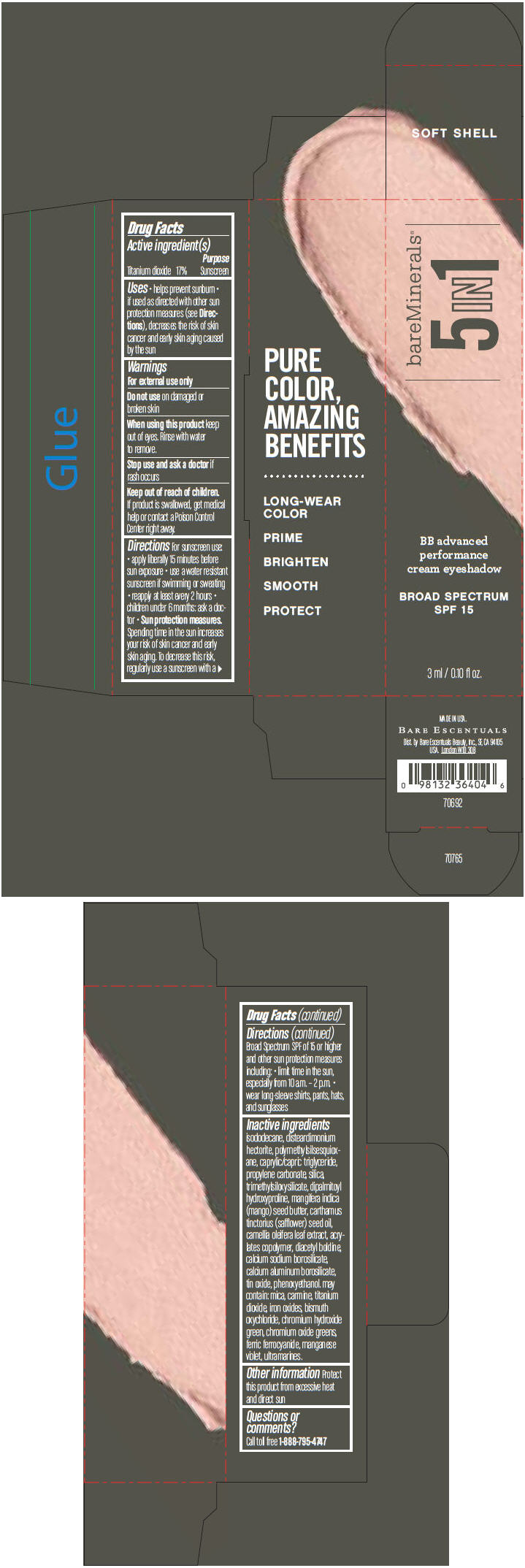
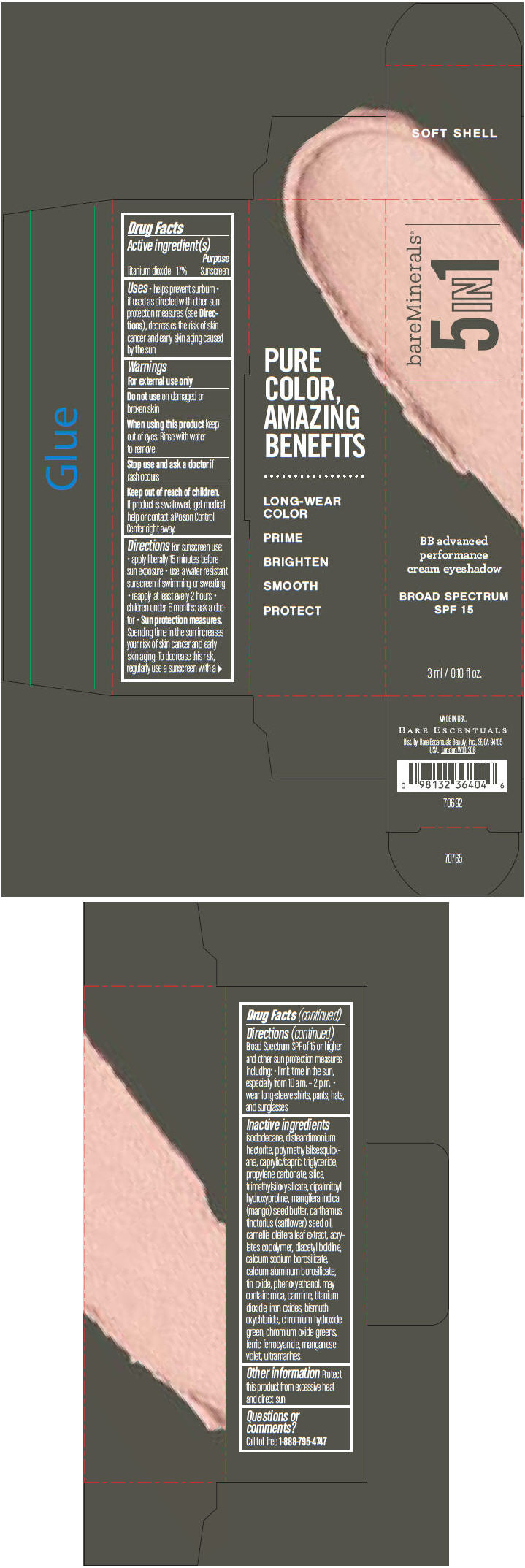
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 SOFT SHELL- titanium dioxide cream
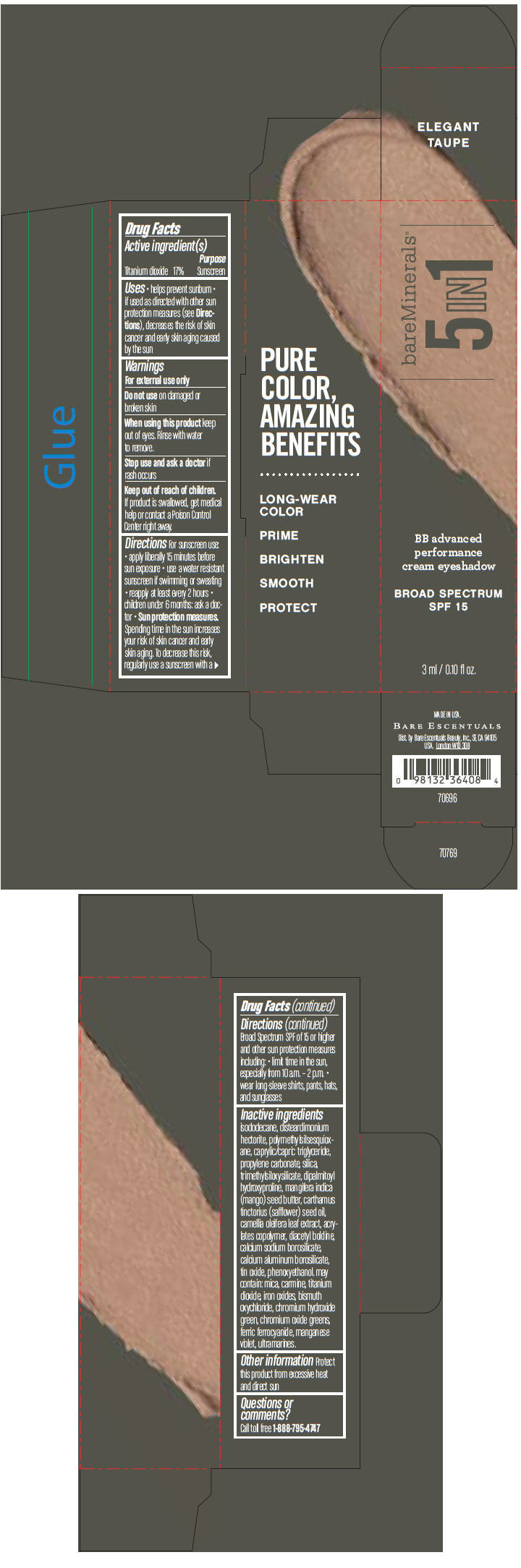
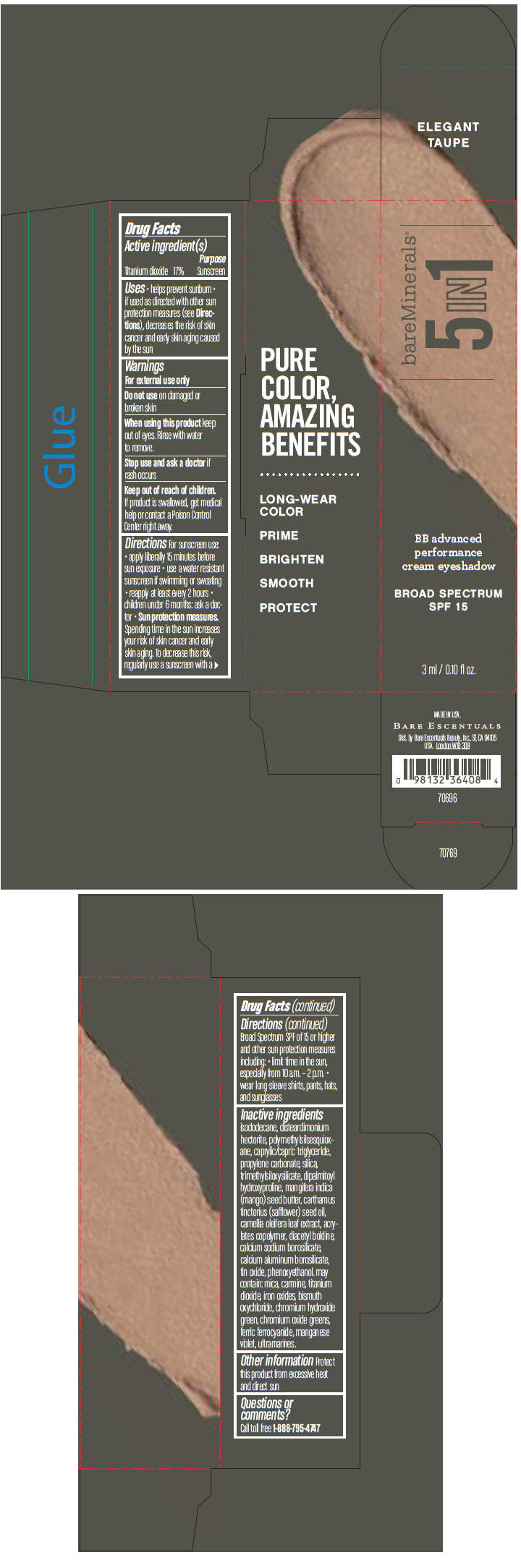
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 ELEGANT TAUPE- titanium dioxide cream
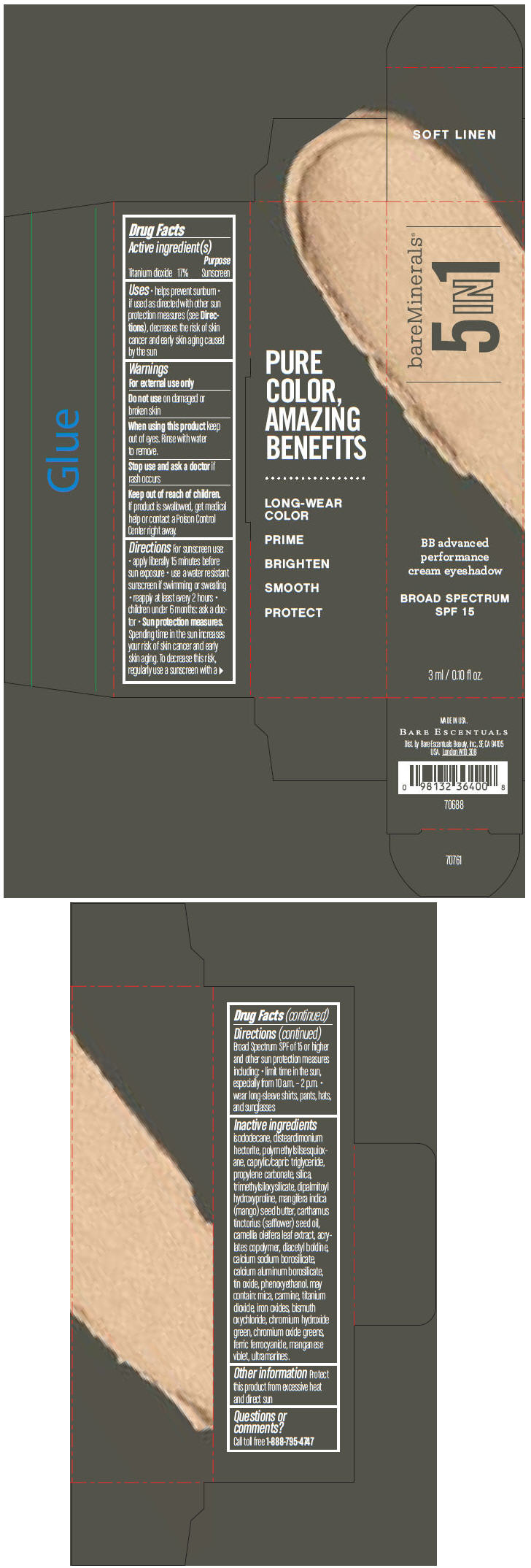
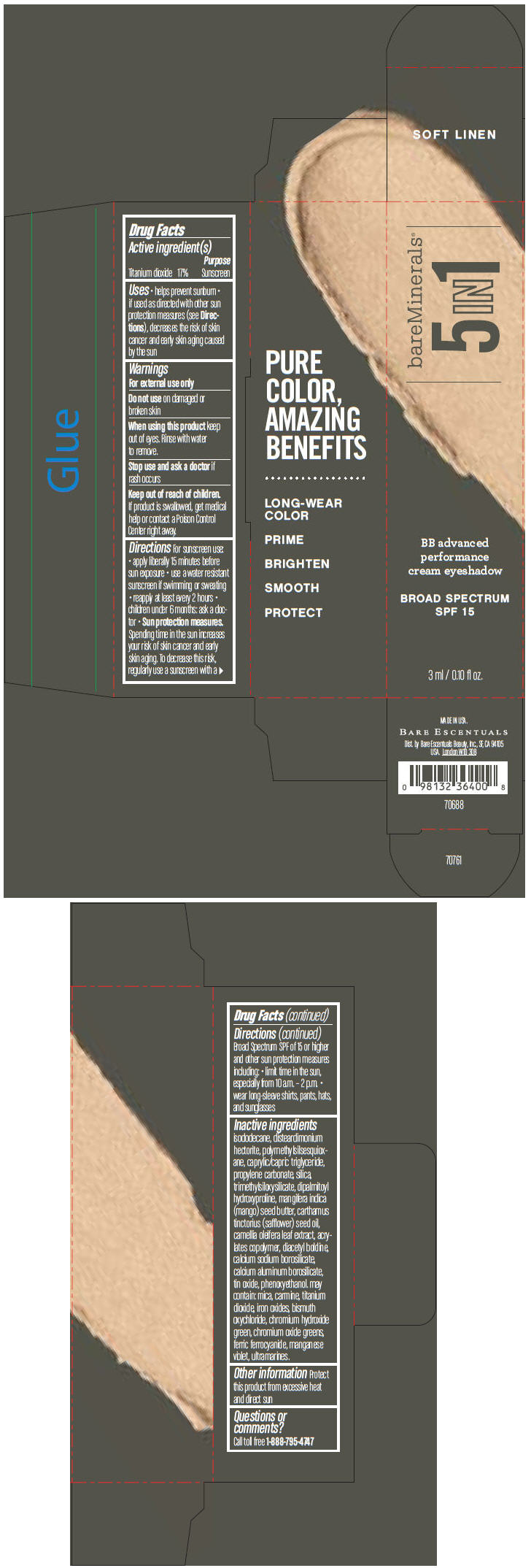
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 SOFT LINEN- titanium dioxide cream
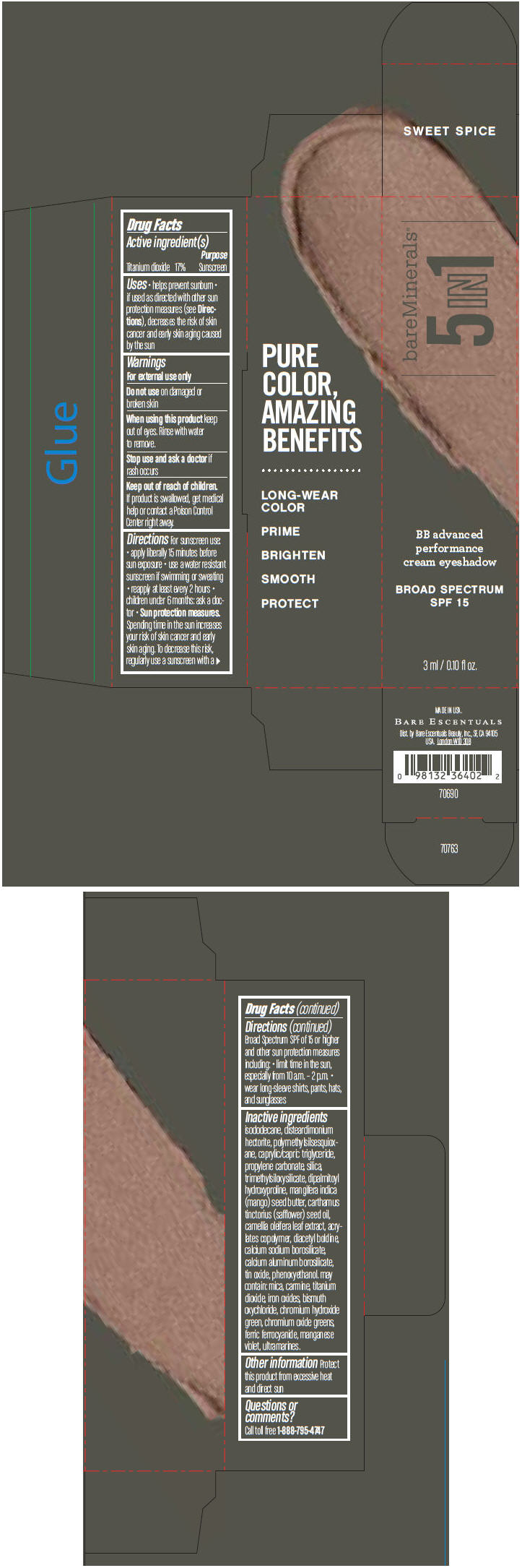
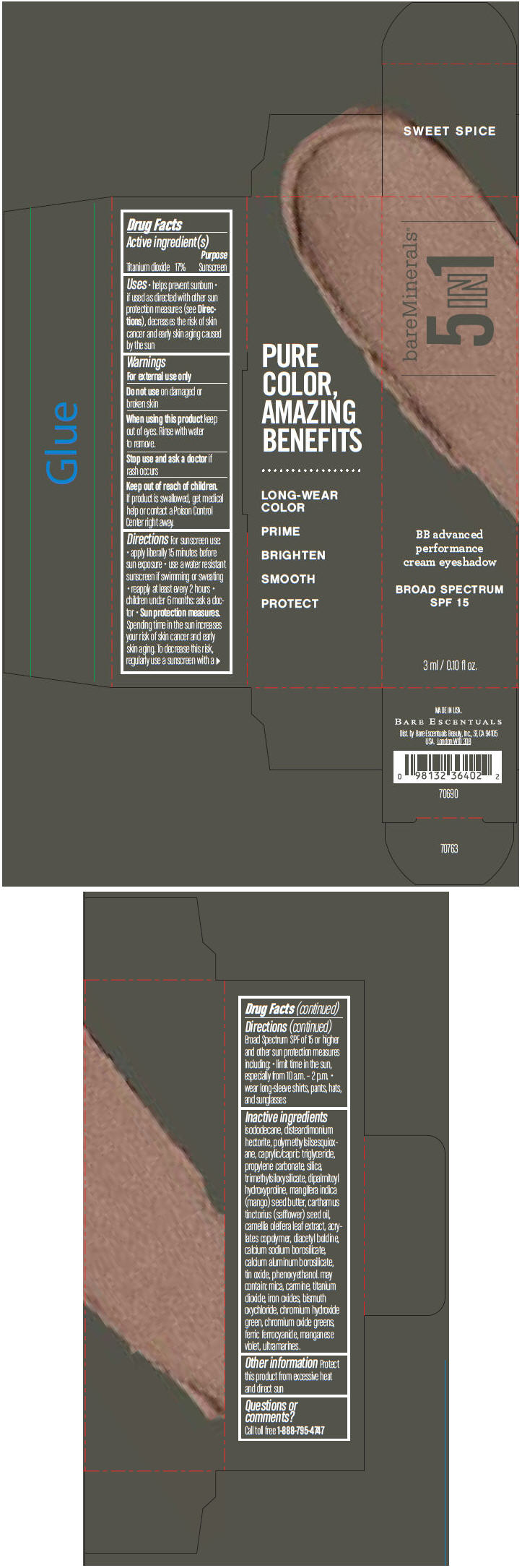
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 SWEET SPICE- titanium dioxide cream
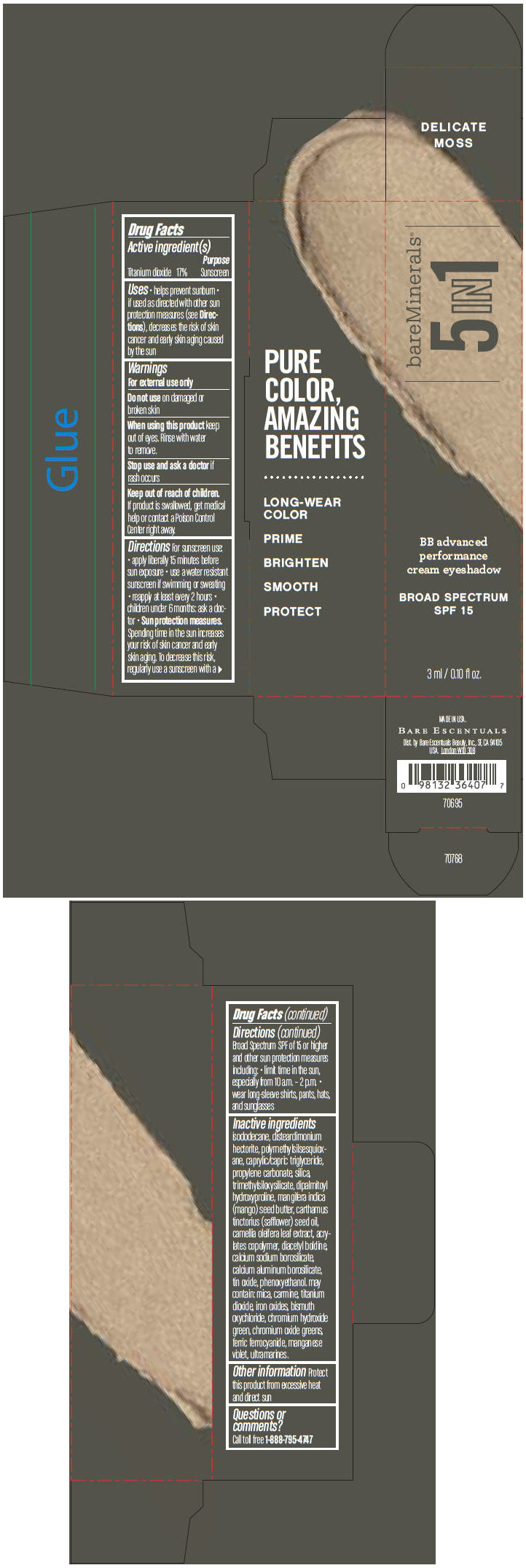
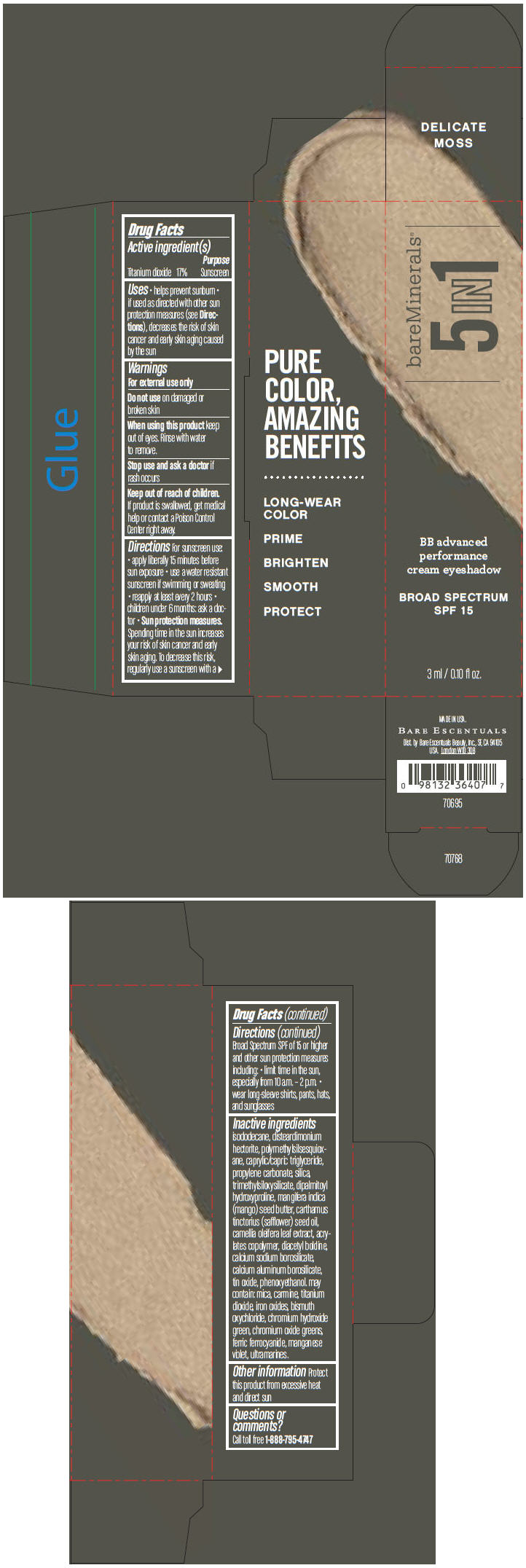
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 DELICATE MOSS- titanium dioxide cream
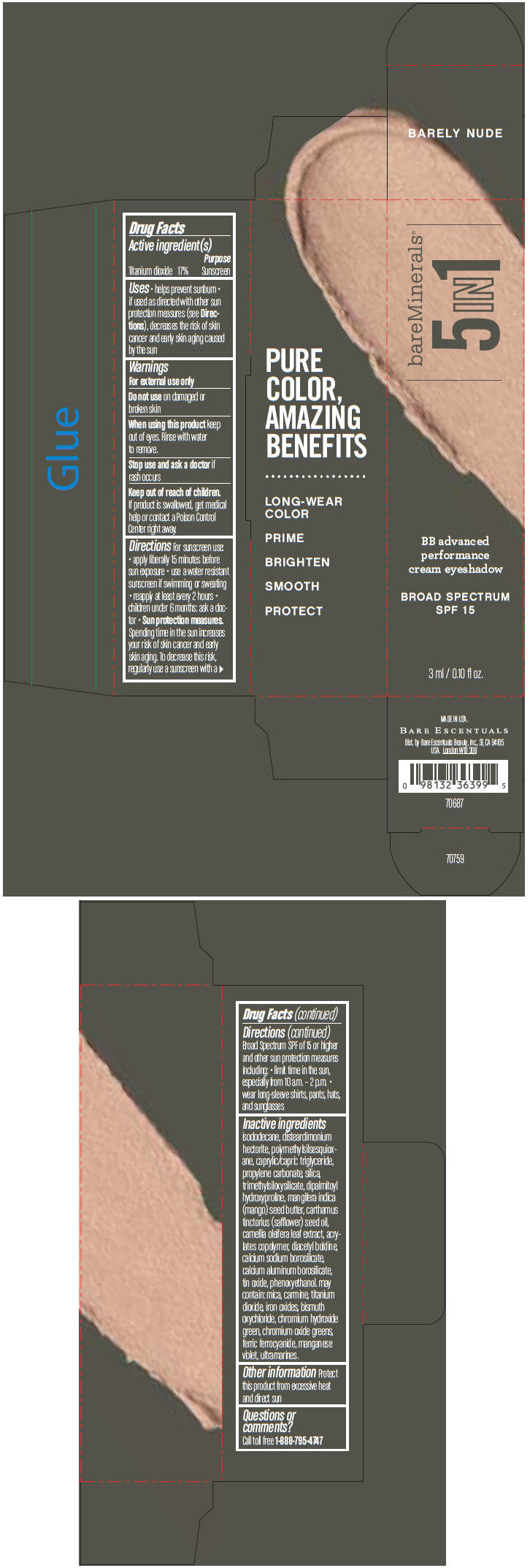
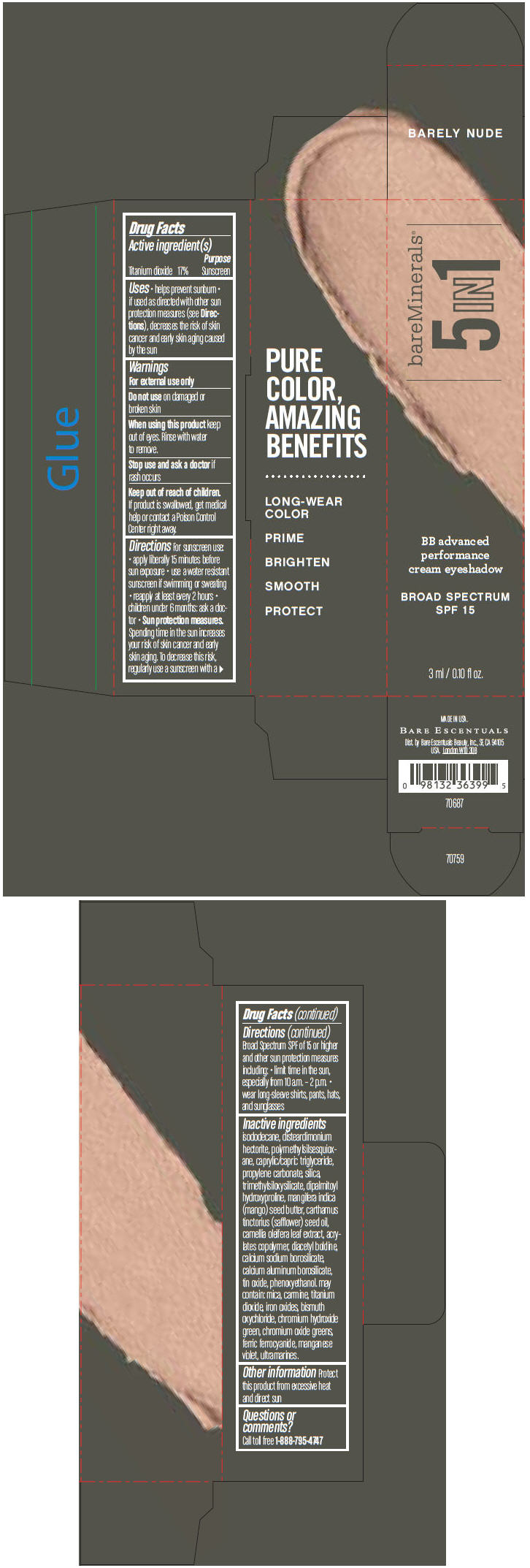
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 BARELY NUDE- titanium dioxide cream
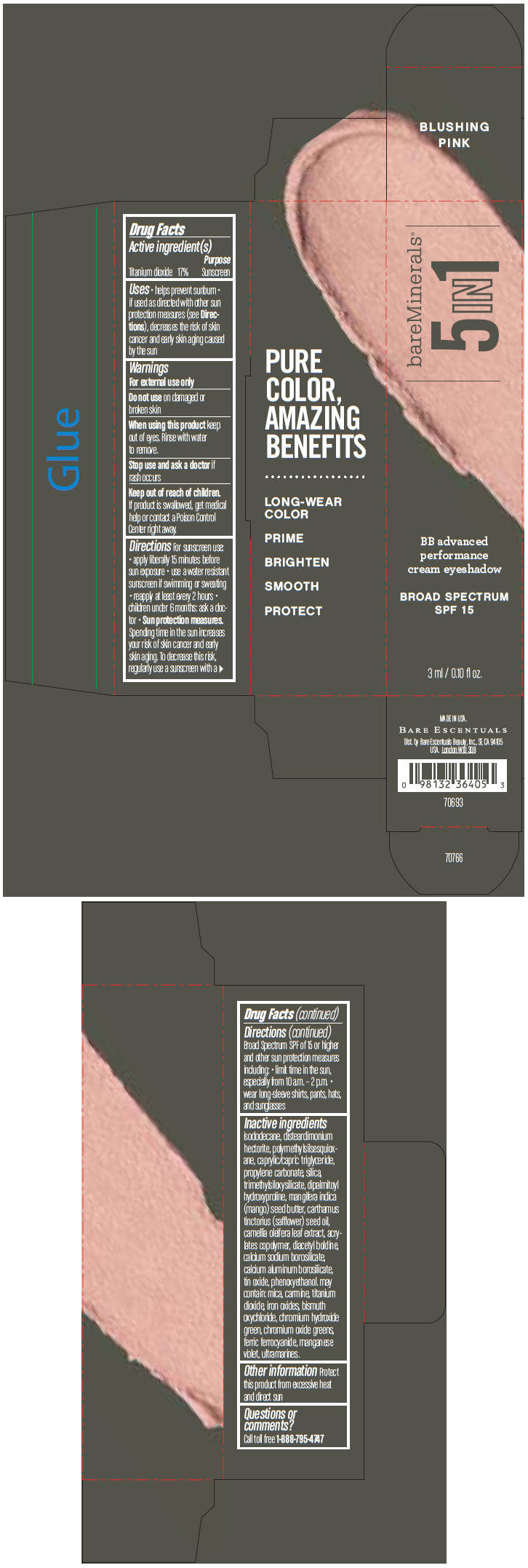
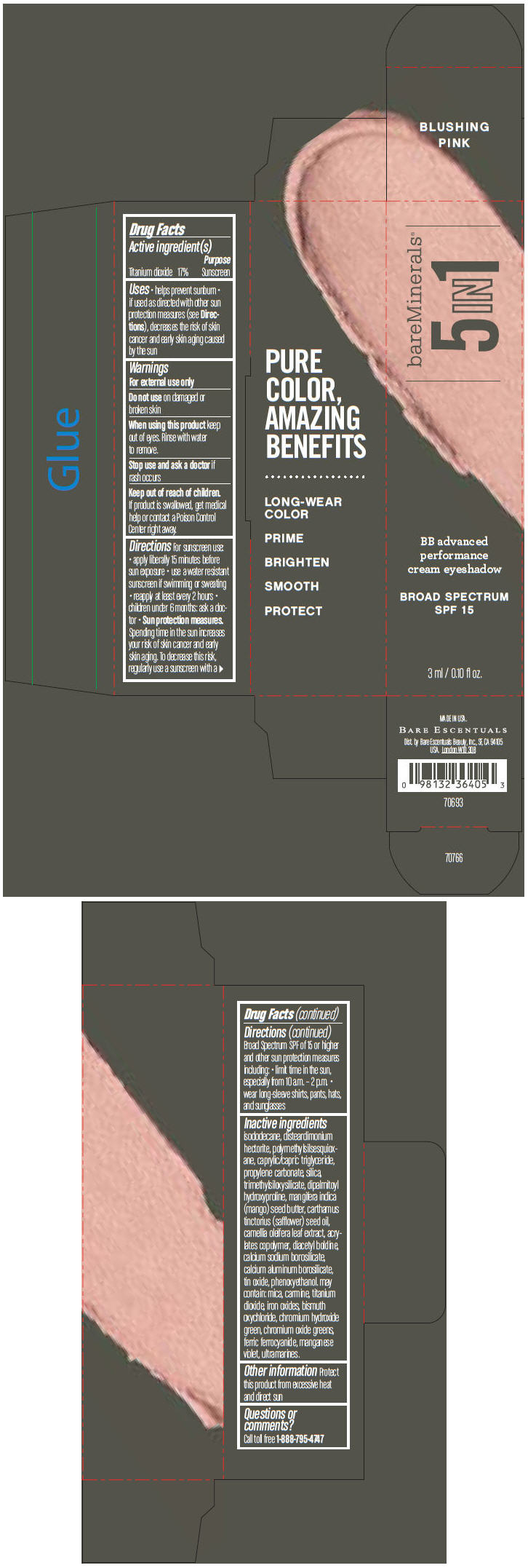
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 BLUSHING PINK- titanium dioxide cream
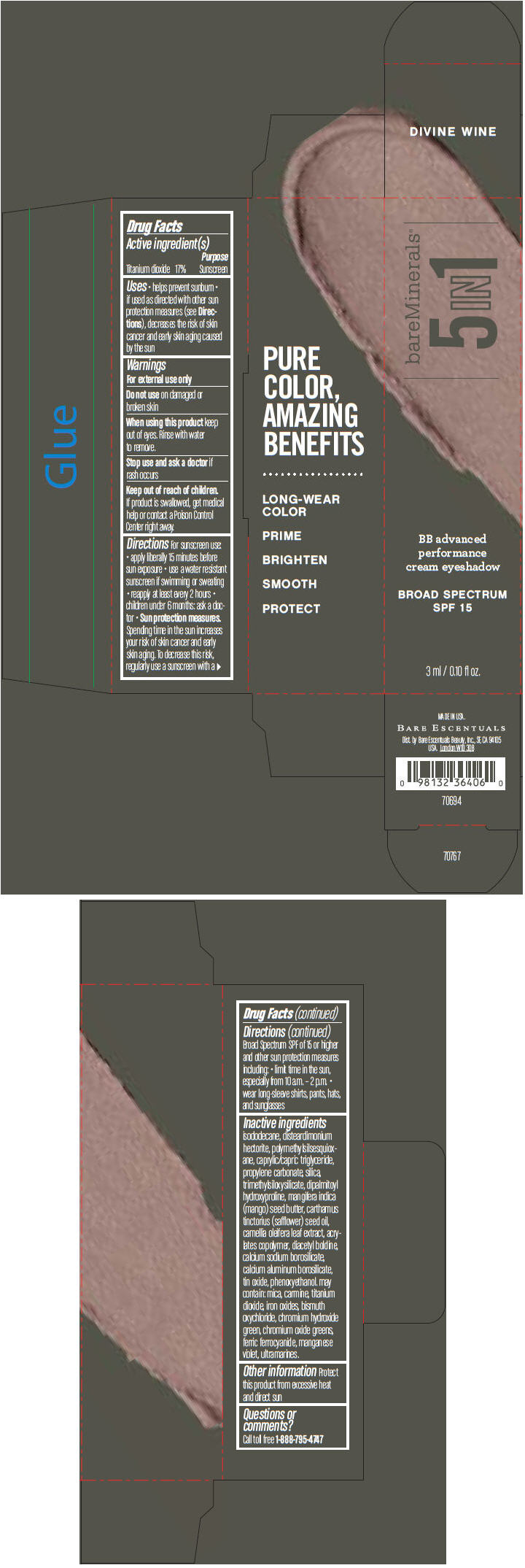
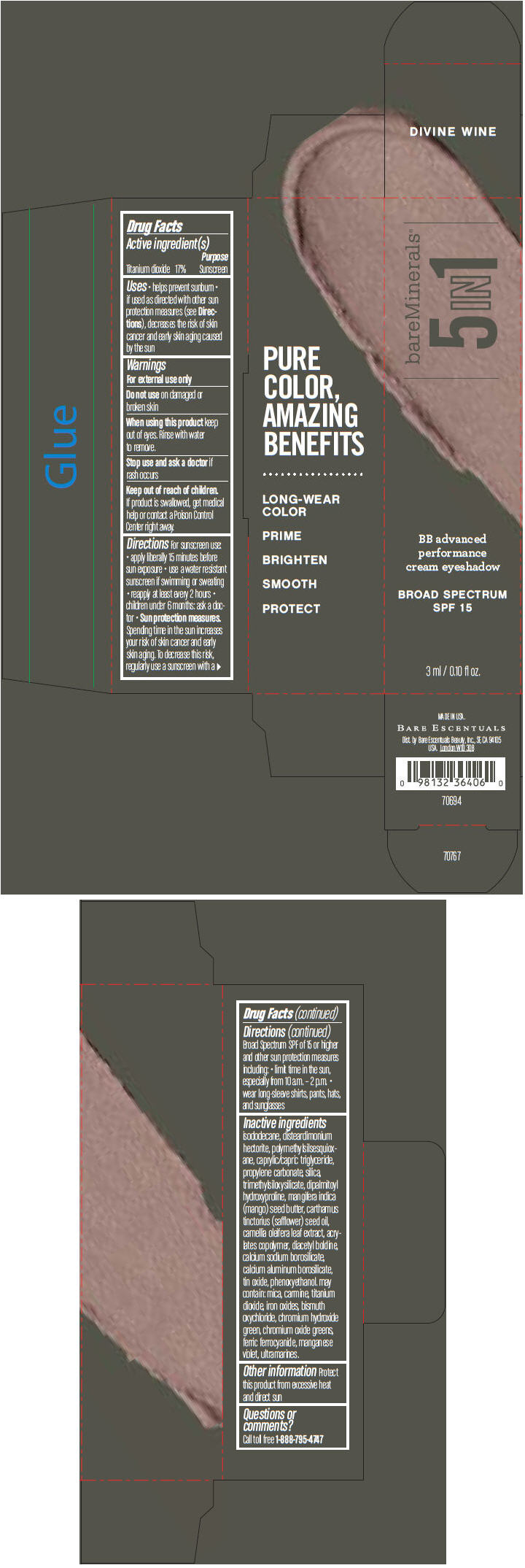
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 DIVINE WINE- titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 98132-700-01, 98132-701-01, 98132-702-01, 98132-703-01, view more98132-704-01, 98132-705-01, 98132-706-01, 98132-707-01, 98132-708-01, 98132-709-01 - Packager: Bare Escentuals Beauty, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 28, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient(s)
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: ask a doctor
-
Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
-
Inactive ingredients
isododecane, disteardimonium hectorite, polymethylsilsesquioxane, caprylic/capric triglyceride, propylene carbonate, silica, trimethylsiloxysilicate, dipalmitoyl hydroxyproline, mangifera indica (mango) seed butter, carthamus tinctorius (safflower) seed oil, camellia oleifera leaf extract, acrylates copolymer, diacetyl boldine, calcium sodium borosilicate, calcium aluminum borosilicate, tin oxide, phenoxyethanol. may contain: mica, carmine, titanium dioxide, iron oxides, bismuth oxychloride, chromium hydroxide green, chromium oxide greens, ferric ferrocyanide, manganese violet, ultramarines.
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Candlelit Peach
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Radiant Sand
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Soft Shell
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Elegant Taupe
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Soft Linen
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Sweet Spice
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Delicate Moss
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Barely Nude
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Blushing Pink
- PRINCIPAL DISPLAY PANEL - 3 ml Tube Carton - Divine Wine
-
INGREDIENTS AND APPEARANCE
BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 CANDLELIT PEACH
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-700 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-700-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 RADIANT SAND
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-701 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-701-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 SOFT SHELL
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-702 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-702-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 ELEGANT TAUPE
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-703 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-703-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 SOFT LINEN
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-704 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-704-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 SWEET SPICE
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-705 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-705-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 DELICATE MOSS
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-706 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-706-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 BARELY NUDE
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-707 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-707-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 BLUSHING PINK
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-708 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-708-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 BAREMINERALS 5-IN-1 BB ADVANCED PERFORMANCE EYESHADOW BROAD SPECTRUM SPF 15 DIVINE WINE
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-709 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 170 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPYLENE CARBONATE (UNII: 8D08K3S51E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPALMITOYL HYDROXYPROLINE (UNII: E6AHA53N1H) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SAFFLOWER OIL (UNII: 65UEH262IS) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) DIACETYL BOLDINE (UNII: 37727Z7M0I) CALCIUM ALUMINUM BOROSILICATE (UNII: 3JRB8A35M0) STANNIC OXIDE (UNII: KM7N50LOS6) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) CHROMIC OXIDE (UNII: X5Z09SU859) FERRIC FERROCYANIDE (UNII: TLE294X33A) MANGANESE VIOLET (UNII: 72M48QQV8Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-709-01 1 in 1 CARTON 1 3 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 03/01/2014 Labeler - Bare Escentuals Beauty, Inc. (087008363)