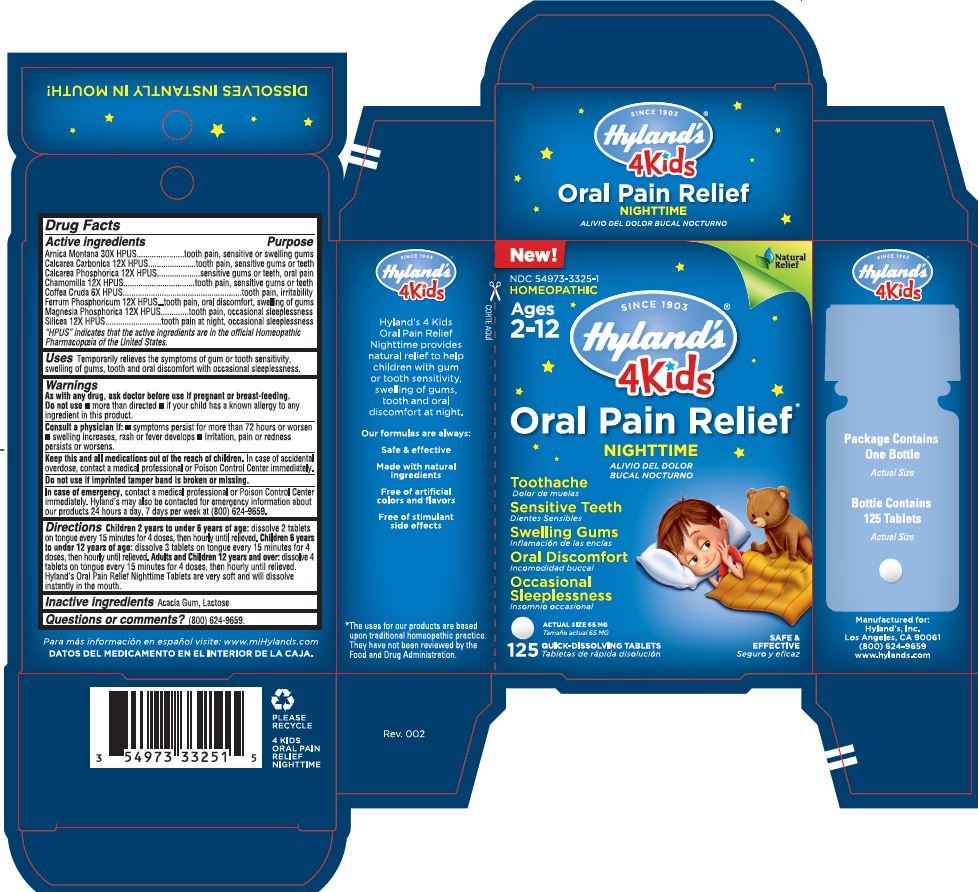
Label: 4 KIDS ORAL PAIN RELIEF NIGHTTIME- arnica montana,tribasic calcium phosphate,silicon dioxide,matricaria chamomilla,ferrum phosphoricum,arabica coffee bean,magnesium phosphate, dibasic trihydrate and oyster shell calcium carbonate, crude tablet
- NDC Code(s): 54973-3325-1, 54973-3325-3
- Packager: Hyland's Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active Ingredients Purpose Arnica Montana 30X HPUS
tooth pain, sensitive or swelling gums
Calcarea Carbonica 12X HPUS
tooth pain, sensitive gums or teeth
Calcarea Phosphorica 12X HPUS
sensitive gums or teeth, oral pain
Chamomilla 12X HPUS
tooth pain, sensitive gums or teeth
Coffea Cruda 6X HPUS
tooth pain, irritability, oral pain
Ferrum Phosphoricum 12X HPUS
tooth pain, oral discomfort, swelling of gums
Magnesia Phosphoricum 12X HPUS
tooth pain, occasional sleeplessness
Silicea 12X HPUS
tooth pain at night, occcasional sleeplessness
"HPUS" indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
- Uses
-
WARNINGS
As with any drug, ask doctor before use if pregnant or breast-feeding.
Do not use • more than directed • if your child has known allergy to any ingredient in this product.
Consult a physician if:
• symptoms persist for more than 72 hours or worsen
• swelling increases, rash or fever develops
• Irritation, pain or redness persist or worsens
-
Directions
Children 2 years to under 6 years of age: dissolve 2 tablets on tongue every 15 minutes for 4 doses, then hourly until relieved.
Children 6 years to under 12 years of age: dissolve 3 tablets on tongue every 15 minutes for 4 doses, then hourly until relieved.
Adults and Children 12 years and over: dissolve 4 tablets on tongue every 15 minutes for 4 doses, then hourly until relieved.
Hyland's Oral Pain Relief Nighttime Tablets are very soft and will dissolve instantly in the mouth.
- INACTIVE INGREDIENT
- Questions or comments?
- PURPOSE
- PRICIPAL DISPLAY PANEL - 125 TABLET CARTON
-
INGREDIENTS AND APPEARANCE
4 KIDS ORAL PAIN RELIEF NIGHTTIME
arnica montana,tribasic calcium phosphate,silicon dioxide,matricaria chamomilla,ferrum phosphoricum,arabica coffee bean,magnesium phosphate, dibasic trihydrate and oyster shell calcium carbonate, crude tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-3325 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] in 1 g MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 12 [hp_X] in 1 g ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 6 [hp_X] in 1 g FERRUM PHOSPHORICUM (UNII: 91GQH8I5F7) (FERRUM PHOSPHORICUM - UNII:91GQH8I5F7) FERRUM PHOSPHORICUM 12 [hp_X] in 1 g SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] in 1 g ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_X] in 1 g TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (PHOSPHATE ION - UNII:NK08V8K8HR) TRIBASIC CALCIUM PHOSPHATE 12 [hp_X] in 1 g MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 12 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color white (Of White) Score no score Shape ROUND Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3325-1 1 in 1 CARTON 08/18/2017 1 125 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:54973-3325-3 6 g in 1 PACKET; Type 0: Not a Combination Product 08/18/2017 12/16/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/18/2017 Labeler - Hyland's Inc. (008316655) Establishment Name Address ID/FEI Business Operations Hyland's Inc. 008316655 manufacture(54973-3325) , pack(54973-3325) , label(54973-3325)