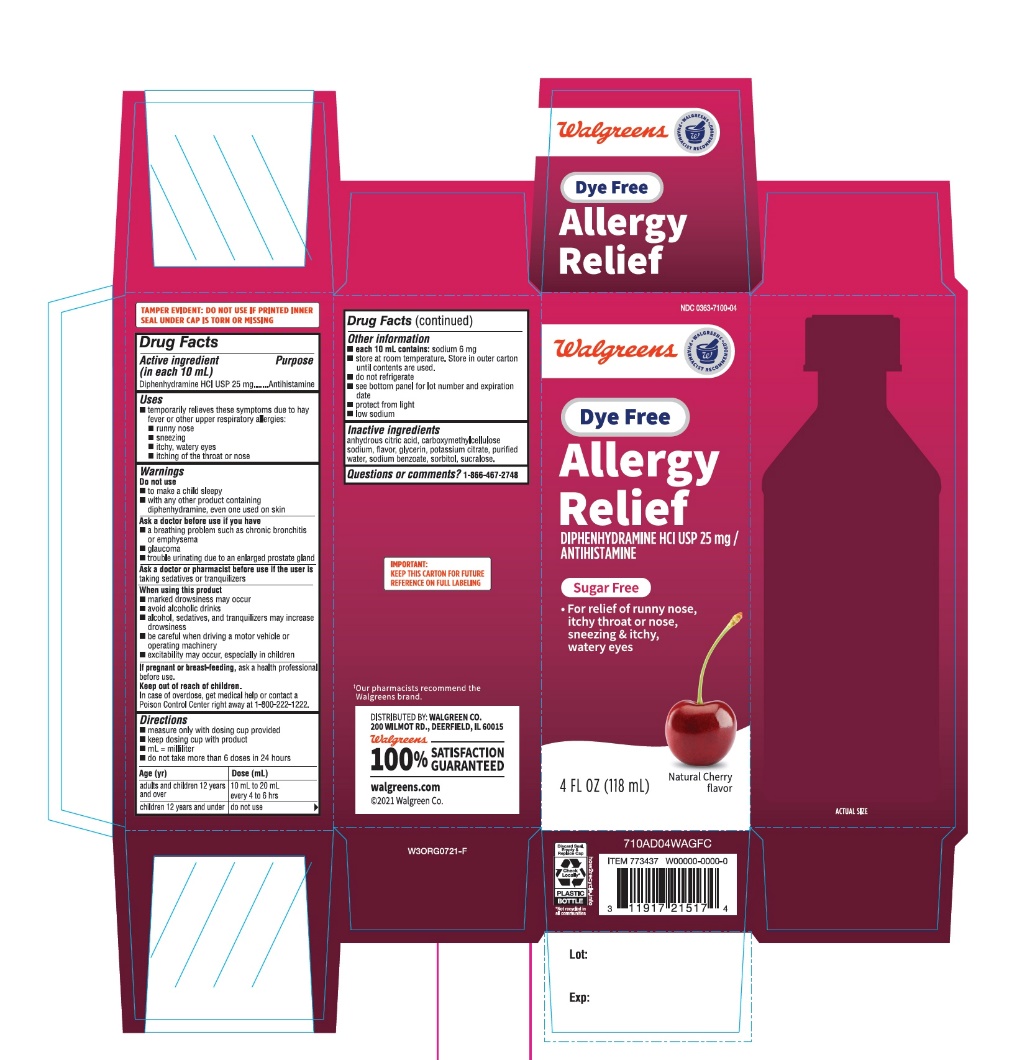
Label: ADULT ALLERGY RELIEF DYE FREE- diphenhydramine hydrochloride solution
- NDC Code(s): 0363-7100-04
- Packager: WALGREEN COMPANY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
- When using this product
- Keep out of reach of children
- Directions
- Other information
- Inactive ingredients
-
Package Label-Principal Display Panel 4 Fl Oz (118 Ml Bottle)
NDC 0363-7100-04
Dye-Free
AdultAllergyRelief
Diphenhydramine HCl | Antihistamine
For Relief of:- •
- Runny nose
- •
- Itchy, watery eyes
- •
- Sneezing and Itchy Watery Eyes
Sugar Free
Cherry FlavorNaturally and Artificially Flavored
4 FL OZ (118 mL)†Our pharmacists recommend the Walgreens brand.
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
Walgreens
100% SATISFACTION GURANTEED
Walgreens.com
@2021 Walgreens Co.
IMPORTANT: KEEP THIS CARTON FOR FUTURE REFERENCE ON FULL LABELING.
-
INGREDIENTS AND APPEARANCE
ADULT ALLERGY RELIEF DYE FREE
diphenhydramine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-7100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) CHERRY (UNII: BUC5I9595W) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color YELLOW (Colorless to Pale Yellow) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-7100-04 1 in 1 CARTON 01/30/2020 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/30/2020 Labeler - WALGREEN COMPANY (008965063)