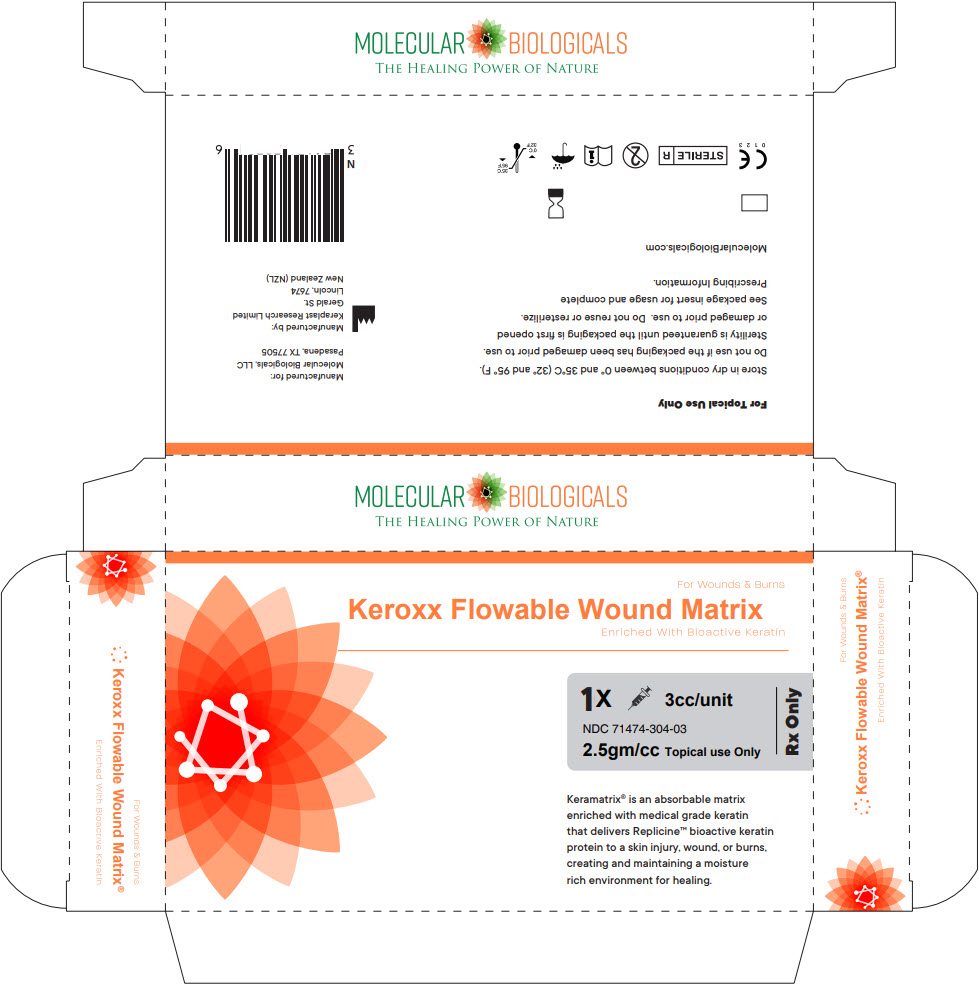
Label: KEROXX- amino acids, hair keratin gel
- NHRIC Code(s): 71474-304-03
- Packager: Molecular Biologicals, LLC
- Category: PRESCRIPTION MEDICAL DEVICE LABEL
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated November 30, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
- DESCRIPTION
- INDICATIONS FOR USE
-
DIRECTIONS FOR USE
(NOTE: ALWAYS HANDLE USING ASEPTIC TECHNIQUE)
WOUND BED PREPARATION & APPLICATION DRESSING CHANGES & REAPPLICATIONS - 1.
- Prepare wound area using standard debridement methods to ensure the wound is free of debris and devitalized tissue. The wound may be surgically debrided to ensure the wound edges contain viable tissue. A small influx of blood may be permitted into the wound.
- 2.
- Remove the tip cap from the 3cc syringe.
- 3.
- Attach the flexible injector tip securely to the syringe.
- 4.
- Slowly dispense Keroxx® Flowable Wound Matrix into the wound area. Avoid pressing tip directly against the wound bed to ensure proper flow of Keroxx® from the flexible injector. For easily visible wound areas a thin 1-3mm film covering is adequate.
- 5.
- After application, use an appropriate non-adherent occlusive dressing. Change dressing as necessary.
- 6.
- Frequency of dressing changes typically occur every 2-5 days, or as determined by the healthcare provider.
- 7.
- Once wound is cleansed using proper technique Keroxx® Flowable Wound Matrix may be reapplied.
- 8.
- Proceed with reapplication of appropriate non-adherent occlusive secondary dressing.
- 9.
- If clinical signs of infection, excessive redness, blistering or suspected allergic reaction occurs the Keroxx should not be reapplied and proper notification and documentation should be done.
- CONTRADICATIONS
- PRECAUTIONS
- STORAGE
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
Keroxx®
3cc syringe/unit
NDC 71474-304-03Manufactured for:
Molecular Biologicals, LLC
Pasadena, TX 77505 | 1-844-793-9933
By Keraplast Research, Ltd
Gerald St. Lincoln, 7674 NZKeroxx® is a registered trademark of Molecular Biologicals, LLC.
The product incorporates patented and /or patent pending technologies owned by Molecular Biologicals, LLC.Issue Date 01Jan17
DWG005/9 -
PRINCIPAL DISPLAY PANEL - 3 cc Syringe Box
For Wounds & Burns
Keroxx Flowable Wound Matrix
Enriched With Bioactive Keratin1X 3cc/unit
NDC 71474-304-03
2.5gm/cc Topical use Only
Rx OnlyKeramatrix® is an absorbable matrix
enriched with medical grade keratin
that delivers Replicine™ bioactive keratin
protein to a skin injury, wound, or burns,
creating and maintaining a moisture
rich environment for healing.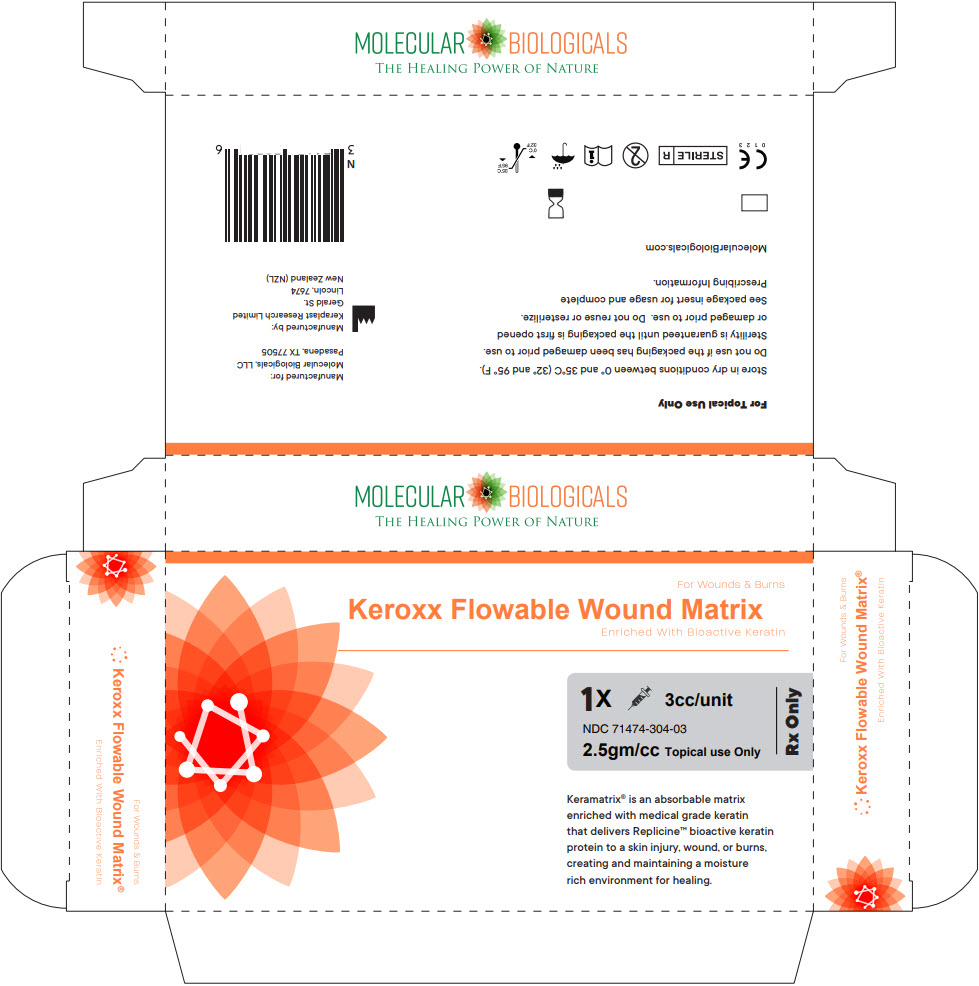
-
INGREDIENTS AND APPEARANCE
KEROXX
dressing, wound, drug gelProduct Information Product Type PRESCRIPTION MEDICAL DEVICE Item Code (Source) NHRIC:71474-304 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMINO ACIDS, HAIR KERATIN (UNII: G46579QK1M) (AMINO ACIDS, HAIR KERATIN - UNII:G46579QK1M) AMINO ACIDS, HAIR KERATIN 100 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:71474-304-03 1 in 1 BOX 1 7.5 g in 1 SYRINGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date EXEMPT DEVICE FRO 02/11/2017 Labeler - Molecular Biologicals, LLC (079518915)