Label: RUGBY BARRIER CREAM- barrier cream cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 0536-1204-73 - Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 3, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
-
Uses
Multipurpose moisture barrier that aids in protecting irritated skin conditions in the perineum, buttocks, lower abdomen and inner thigh due to moisture, occlusion, chafing or continued contact with urine or feces.
Helps relieve the local itching, discomfort, pain, burning and irritation associated with anorectal disorders and hemorroids.May provide a cooling sensation.
-
Warnings
Stop use and consult a doctor
•in case of bleeding
•if conditions worsenor deos not improve within 7 days<Do not exceed recommended daily dosage unless directed by a doctor. Do not put product into rectum by using fingers or any mechanical device or applicatorAllergy Alert:Certain persons can develop allergic reactions to this product. If the symptoms develop or increase,discontinue use and consult a doctor. >
- Directions
- Other information
- Inactive ingredients
- Principal Display Panel - Tube Label
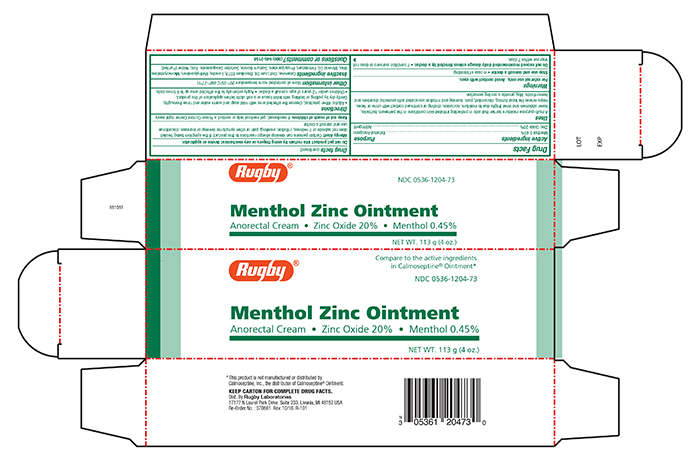
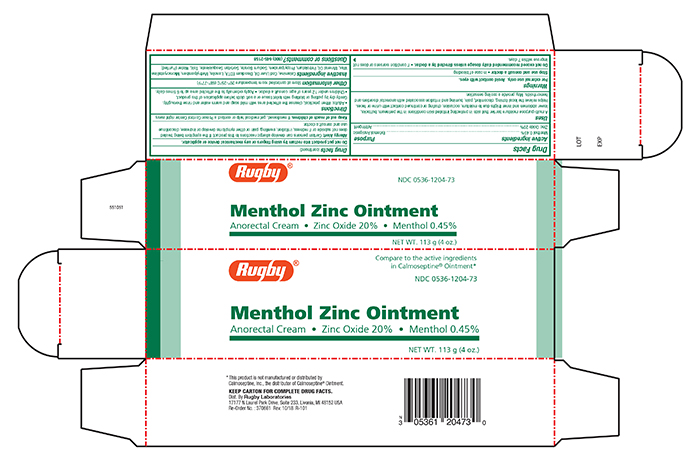
- Principal Display Panel - Carton
-
INGREDIENTS AND APPEARANCE
RUGBY BARRIER CREAM
barrier cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1204 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 200 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 4.5 mg in 1 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) COD LIVER OIL (UNII: BBL281NWFG) DISODIUM ETHYLENEDIAMINEDIACETATE (UNII: EQL53S5L0F) LANOLIN (UNII: 7EV65EAW6H) METHYLPARABEN (UNII: A2I8C7HI9T) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM BORATE (UNII: 91MBZ8H3QO) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) TALC (UNII: 7SEV7J4R1U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1204-73 1 in 1 CARTON 05/06/2019 1 113 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 05/03/2019 Labeler - Rugby Laboratories (079246066) Registrant - Sheffield Pharmaceuticals, LLC (151177797) Establishment Name Address ID/FEI Business Operations Sheffield Pharmaceuticals, LLC 151177797 MANUFACTURE(0536-1204)