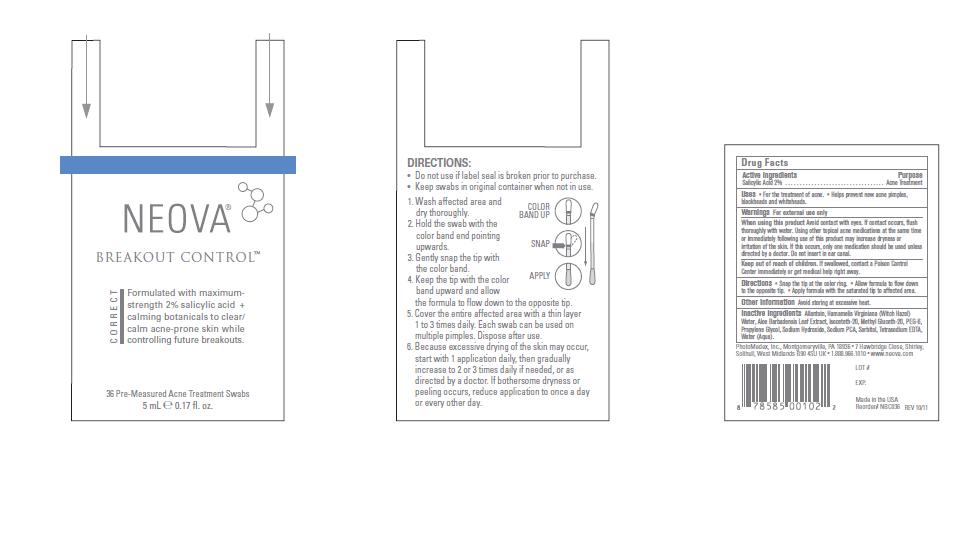
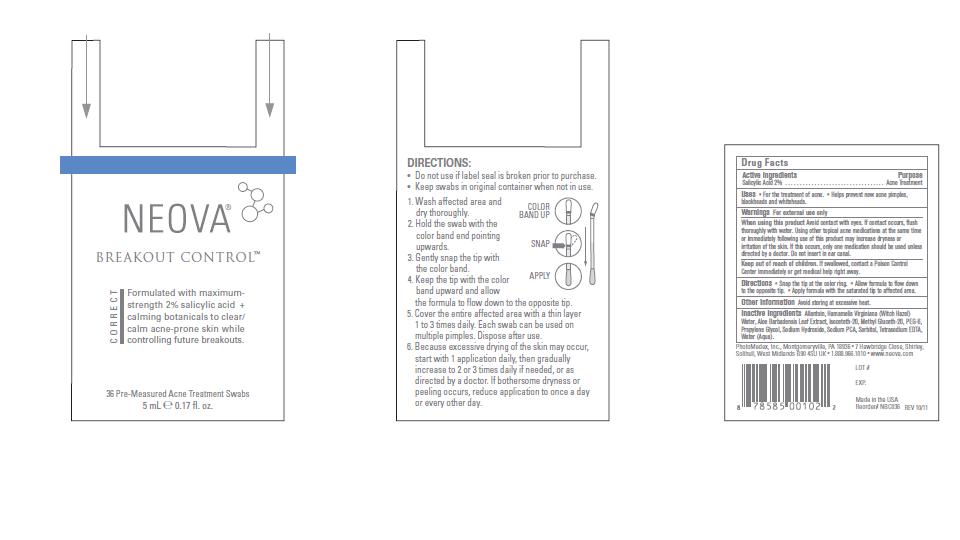
Label: NEOVA BREAKOUT CONTROL- salicylic acid swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 62362-119-05, 62362-119-36 - Packager: PhotoMedex, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 28, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
When using this product
Avoid contact with eyes. If contact occurs, flush
thoroughly with water. Using other topical acne medications at the same time
or immediately following use of this product may increase dryness or
irritation of the skin. If this occurs, only one medication should be used unless
directed by a doctor. Do not insert in ear canal. - Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Image of Box Label
-
INGREDIENTS AND APPEARANCE
NEOVA BREAKOUT CONTROL
salicylic acid swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62362-119 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 2 mL in 100 mL Inactive Ingredients Ingredient Name Strength Allantoin (UNII: 344S277G0Z) Hamamelis Virginiana Leaf Water (UNII: 8FP93ED6H2) Aloe Vera Leaf (UNII: ZY81Z83H0X) Isoceteth-20 (UNII: O020065R7Z) Methyl Gluceth-20 (UNII: J3QD0LD11P) Polyethylene Glycol 300 (UNII: 5655G9Y8AQ) Propylene Glycol (UNII: 6DC9Q167V3) Sodium Hydroxide (UNII: 55X04QC32I) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Sorbitol (UNII: 506T60A25R) Edetate Sodium (UNII: MP1J8420LU) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62362-119-36 36 in 1 BOX 1 NDC:62362-119-05 5 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358B 12/27/2011 Labeler - PhotoMedex, Inc. (054503875) Establishment Name Address ID/FEI Business Operations PhotoMedex, Inc. 054503875 manufacture