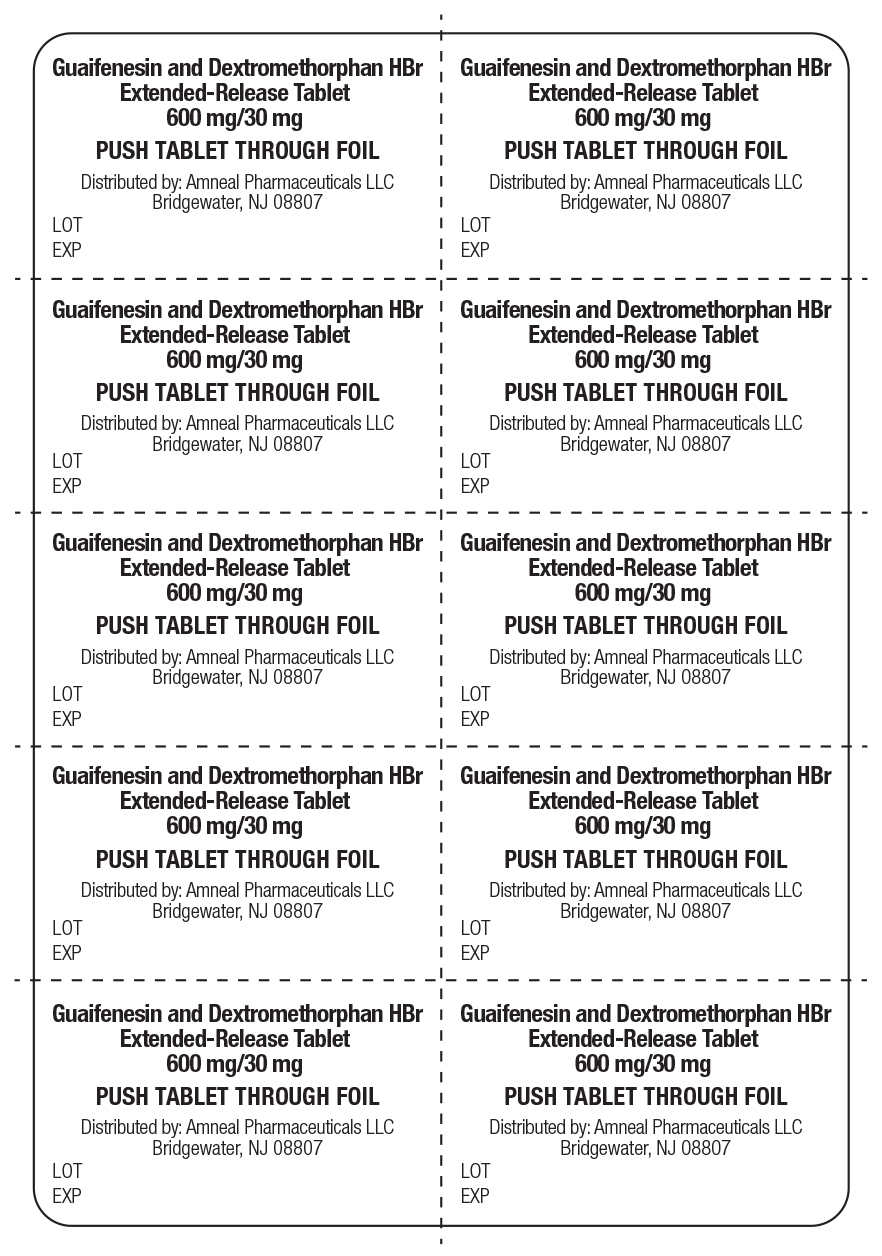
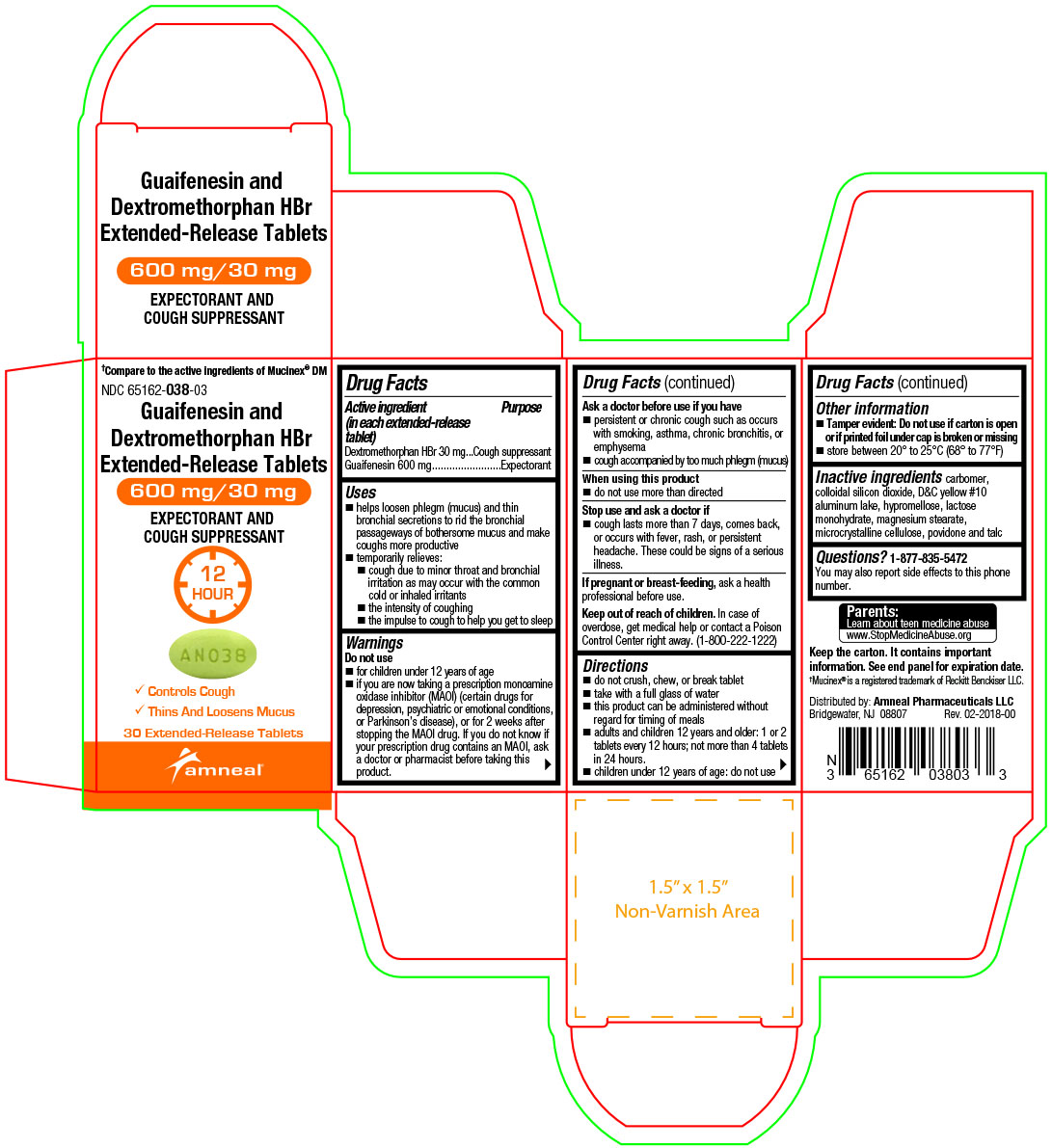
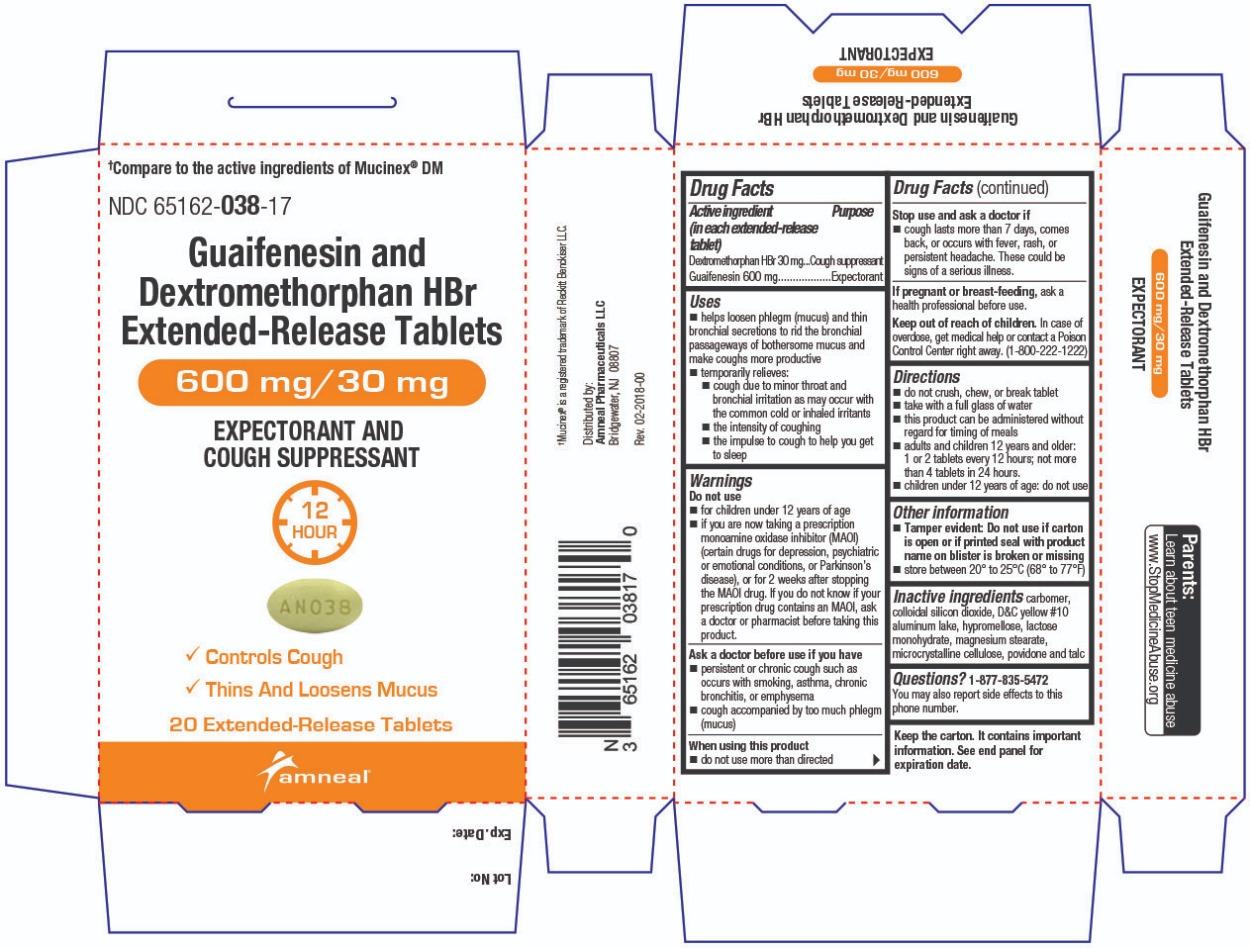
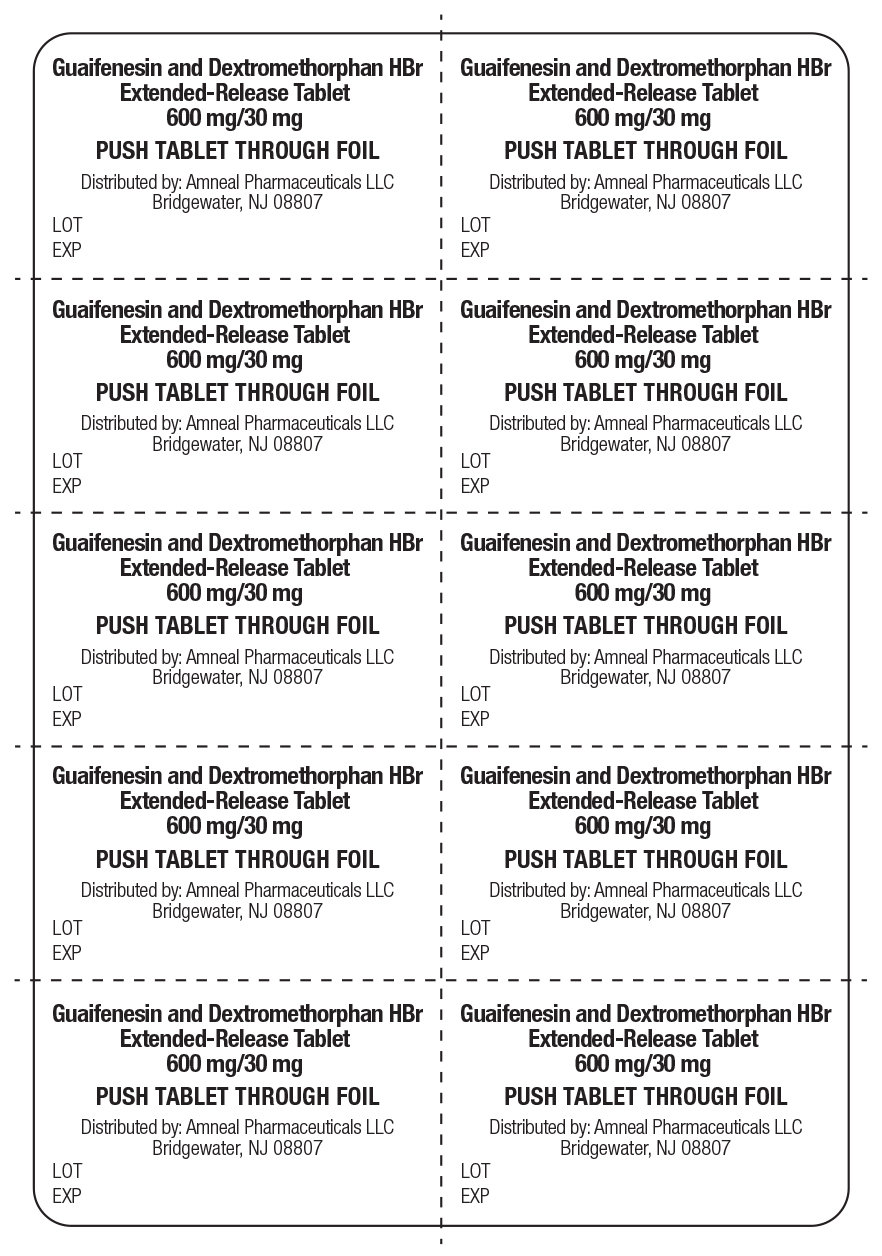
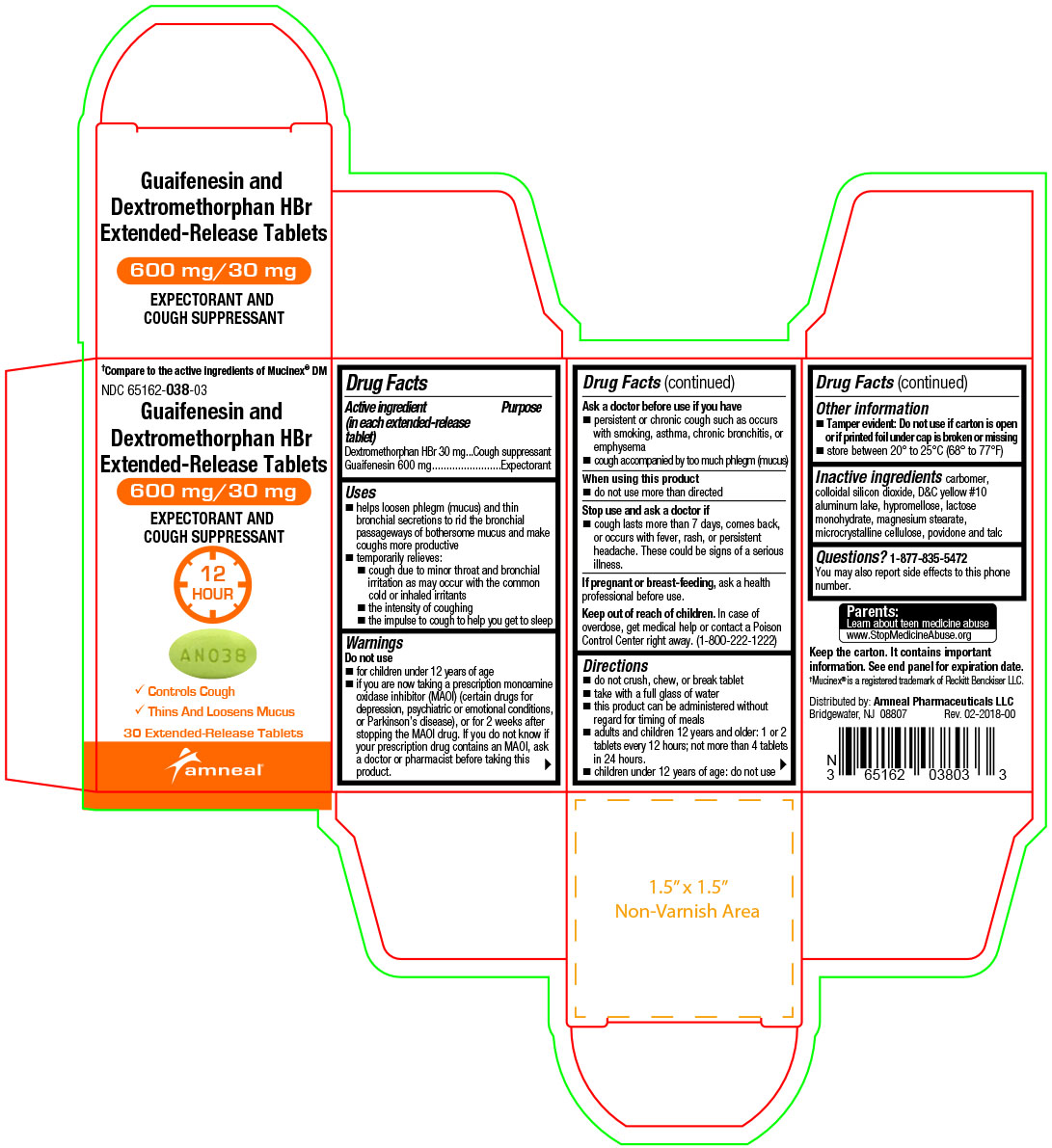
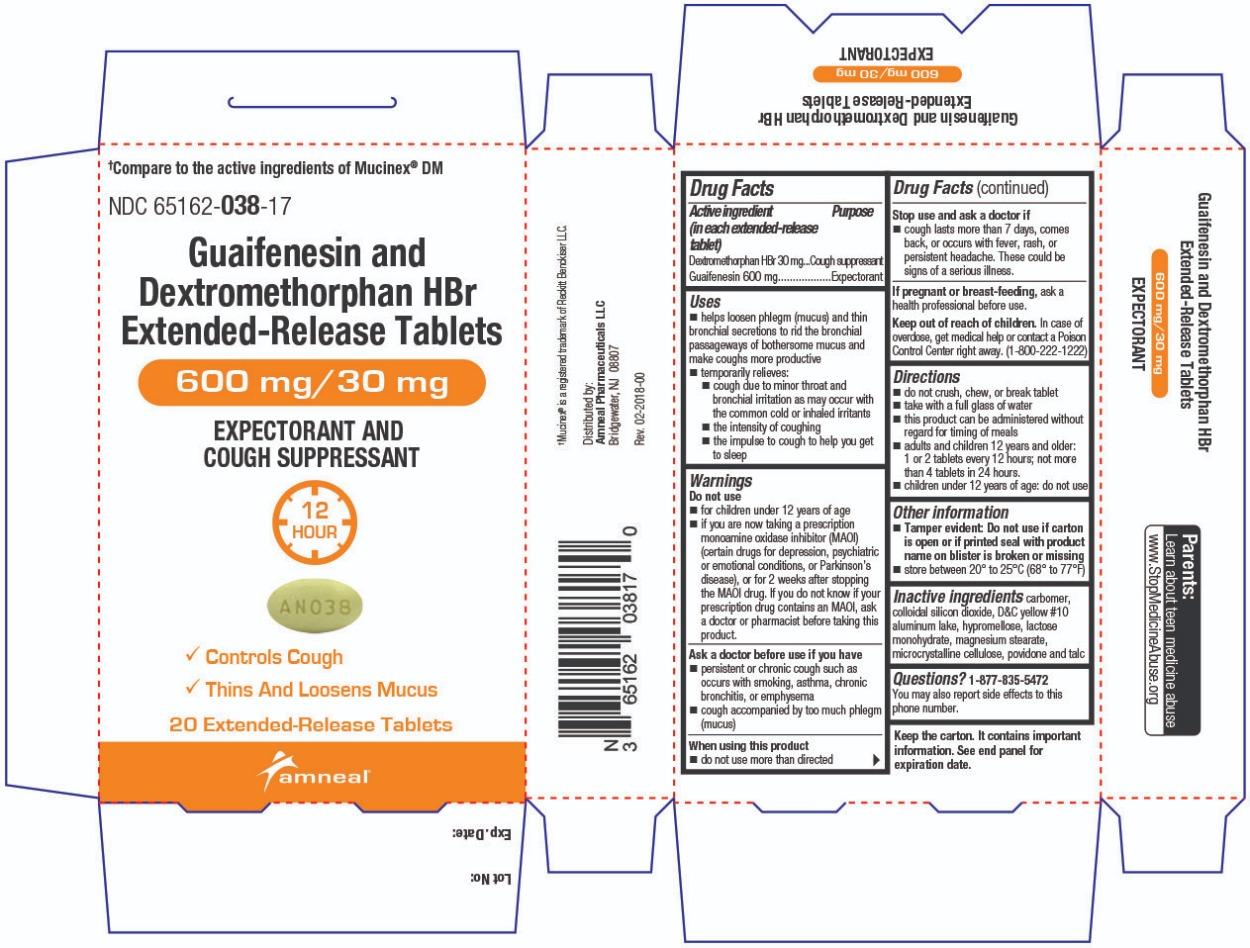
Label: GUAIFENESIN AND DEXTROMETHORPHAN HBR tablet, extended release
- NDC Code(s): 65162-038-03, 65162-038-06, 65162-038-17
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient(s)(in each extended-release tablet)
- Purpose
-
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
-
Warnings
Do not use
- for children under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
- Directions
- Other information
- Inactive ingredients
-
Questions?
1-877-835-5472
You may also report side effects to this phone number.Parents:
Learn about teen medicine abuse
www.StopMedicineAbuse.orgKeep the carton. It contains important information.
See end panel for expiration date.†Mucinex® is a registered trademark of Reckitt Benckiser LLC.
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807Rev. 02-2018-00
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN AND DEXTROMETHORPHAN HBR
guaifenesin and dextromethorphan hbr tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65162-038 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) CARBOMER HOMOPOLYMER TYPE A (UNII: F68VH75CJC) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color yellow (light yellow) Score no score Shape OVAL Size 16mm Flavor Imprint Code AN038 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65162-038-17 2 in 1 CARTON 11/01/2018 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:65162-038-03 1 in 1 CARTON 11/01/2018 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:65162-038-06 1 in 1 CARTON 11/01/2018 3 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209692 11/01/2018 Labeler - Amneal Pharmaceuticals LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals of New York, LLC 123797875 analysis(65162-038) , label(65162-038) , manufacture(65162-038) , pack(65162-038)