Label: EOHILIA- budesonide suspension
- NDC Code(s): 64764-105-00, 64764-105-01, 64764-105-10, 64764-105-60
- Packager: TAKEDA PHARMACEUTICALS AMERICA, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EOHILIA safely and effectively. See full prescribing information for EOHILIA.
EOHILIA™ (budesonide oral suspension)
Initial U.S. Approval: 1997INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Oral suspension: 2 mg/10 mL unit-dose packets. (3)
CONTRAINDICATIONS
Hypersensitivity to budesonide. (4)
WARNINGS AND PRECAUTIONS
- Hypercorticism and Adrenal Axis Suppression: May occur with treatment; monitor for signs and symptoms and consider reducing the dosage. (5.1, 8.6)
- Immunosuppression and Increased Risk of Infection: Increased risk of viral, bacterial, fungal, protozoan, and helminthic infections, including potentially fatal varicella and measles infection. Monitor patients for new or worsening localized or systemic infection, including oropharyngeal and esophageal candidiasis and consider drug discontinuation. Avoid use in patients with fungal infections, Strongyloides infestation, cerebral malaria, and ocular herpes simplex. Screen for hepatitis B infection. (5.2)
- Erosive Esophagitis: Advise patients or caregivers to report new or worsening signs or symptoms of erosive esophagitis; consider endoscopic evaluation as appropriate. (5.3)
- Effect on Growth: Use of corticosteroids may cause a reduction in growth velocity in pediatric patients; monitor growth during treatment. (5.4)
- Symptoms of Steroid Withdrawal in Patients Transferred from Other Systemic Corticosteroids: Taper slowly from corticosteroids with high systemic effects; monitor for withdrawal symptoms and unmasking of allergies (rhinitis, eczema). (5.5)
- Other Corticosteroid Effects: Monitor patients with concomitant conditions where corticosteroids may have unwanted effects (e.g., hypertension, diabetes mellitus). (5.6)
- Kaposi’s Sarcoma: Reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. (5.7)
ADVERSE REACTIONS
Most common adverse reactions (≥2%) are: respiratory tract infection, gastrointestinal mucosal candidiasis, headache, gastroenteritis, throat irritation, adrenal suppression, and erosive esophagitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Hepatic Impairment: Use is not recommended in severe hepatic impairment. Monitor patients with moderate hepatic impairment for signs and/or symptoms of hypercorticism. (5.1, 8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypercorticism and Adrenal Axis Suppression
5.2 Immunosuppression and Increased Risk of Infection
5.3 Erosive Esophagitis
5.4 Effect on Growth
5.5 Symptoms of Steroid Withdrawal in Patients Transferred from Other Systemic Corticosteroids
5.6 Other Corticosteroid Effects
5.7 Kaposi’s Sarcoma
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
EOHILIA™ is indicated for 12 weeks of treatment in adult and pediatric patients 11 years of age and older with eosinophilic esophagitis (EoE).
Limitations of Use
EOHILIA has not been shown to be safe and effective for the treatment of EoE for longer than 12 weeks [see Dosage and Administration (2.1), Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.2 Preparation and Important Administration Instructions
- Do NOT take EOHILIA with food or liquid at the time of ingestion. Wait for at least 30 minutes to eat or drink after taking EOHILIA.
- Administer EOHILIA as follows:
- Do NOT mix EOHILIA with food or liquid.
- Shake the EOHILIA packet for at least 10 seconds prior to opening.
- Squeeze the packet from the bottom to the top directly into the mouth. Repeat 2 to 3 times until the EOHILIA packet is empty.
- Swallow all the EOHILIA suspension.
- Do not eat or drink for 30 minutes after taking EOHILIA. After 30 minutes, rinse mouth with water and spit out the contents without swallowing [see Warnings and Precautions (5.2)].
- Avoid consumption of grapefruit juice for the duration of therapy with EOHILIA [see Drug Interactions (7.1)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
EOHILIA is contraindicated in patients with hypersensitivity to budesonide. Serious hypersensitivity reactions, including anaphylaxis, have occurred with oral budesonide products [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypercorticism and Adrenal Axis Suppression
Systemic effects such as hypercorticism and adrenal axis suppression may occur with use of corticosteroids, including EOHILIA. Monitor patients for signs and symptoms of hypercorticism and adrenal axis suppression and consider reducing the dosage of EOHILIA [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)].
Patients with moderate to severe hepatic impairment (Child-Pugh Class B and C, respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure of oral budesonide. Use is not recommended in patients with severe hepatic impairment (Child-Pugh Class C) and monitoring for signs and/or symptoms of hypercorticism is recommended in patients with moderate hepatic impairment (Child-Pugh Class B) [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
Corticosteroids, including EOHILIA, can reduce the response of the hypothalamus-pituitary-adrenal (HPA) axis to stress. In situations where patients are subject to trauma, surgery, infection, or other stress situations, supplementation with a systemic corticosteroid is recommended.
5.2 Immunosuppression and Increased Risk of Infection
Corticosteroids, including EOHILIA, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids can:
- Reduce resistance to new infections
- Exacerbate existing infections
- Increase the risk of disseminated infections
- Increase the risk of reactivation or exacerbation of latent infections
- Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor patients for the development of infection and consider discontinuation of EOHILIA if the patient develops an infection while on treatment.
Tuberculosis
If corticosteroids are used in patients with latent tuberculosis or tuberculin reactivity, reactivation of tuberculosis may occur. Closely monitor such patients for reactivation while receiving EOHILIA.
Varicella Zoster and Measles Viral Infections
Varicella and measles can have a serious or even fatal course in non-immune pediatric and adult patients taking corticosteroids. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles:
- If an EOHILIA-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
- If an EOHILIA-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Prior to starting treatment with EOHILIA, for patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections
Corticosteroids may exacerbate systemic fungal infections; therefore, avoid EOHILIA use in the presence of such infections.
Amebiasis
Corticosteroids may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating EOHILIA in patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation
Avoid EOHILIA in patients with known or suspected Strongyloides (threadworm) infection. Corticosteroid-induced immunosuppression may lead to Strongyloides superinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Cerebral Malaria
Avoid corticosteroids, including EOHILIA, in patients with cerebral malaria.
Ocular Herpes Simplex
Avoid corticosteroids, including EOHILIA, in patients with active ocular herpes simplex.
Localized Infections
In clinical trials with EOHILIA, localized infections with Candida albicans occurred in the mouth, throat, and esophagus in some subjects [see Adverse Reactions (6.1)]. Do not eat or drink for 30 minutes after taking EOHILIA. After 30 minutes, rinse mouth with water and spit without swallowing [see Dosage and Administration (2.2)]. If oropharyngeal or esophageal candidiasis develops, treat with appropriate local or systemic antifungal therapy and consider discontinuing treatment with EOHILIA.
5.3 Erosive Esophagitis
Erosive esophagitis occurred in subjects who received EOHILIA in a 12-week clinical trial. None of the subjects had erosions at baseline esophagogastroduodenoscopy (EGD), and most were receiving concomitant therapy with a proton pump inhibitor (PPI) during the trial [see Adverse Reactions (6.1)].
Advise patients or caregivers to report new onset or worsening signs or symptoms of erosive esophagitis to their healthcare provider. Consider endoscopic evaluation as appropriate.
5.4 Effect on Growth
Use of corticosteroids may cause a reduction of growth velocity in pediatric patients. Monitor the growth of pediatric patients on EOHILIA. The maximum recommended duration of treatment with EOHILIA is 12 weeks [see Dosage and Administration (2.1)].
5.5 Symptoms of Steroid Withdrawal in Patients Transferred from Other Systemic Corticosteroids
Monitor patients who are transferred from corticosteroid treatment with high systemic effects to corticosteroids with lower systemic availability, such as EOHILIA, since symptoms attributed to withdrawal of steroid therapy, including those of acute adrenal axis suppression or benign intracranial hypertension, may develop. Adrenocortical function monitoring may be required in these patients and the dose of corticosteroid treatment with high systemic effects should be reduced cautiously.
Replacement of systemic corticosteroids with EOHILIA may unmask allergies (e.g., rhinitis and eczema), which were previously controlled by the systemic drug.
5.6 Other Corticosteroid Effects
Monitor patients with hypertension, diabetes mellitus, osteoporosis, peptic ulcer, glaucoma or cataracts, or with a family history of diabetes or glaucoma, or with any other condition where corticosteroids may have unwanted effects.
5.7 Kaposi’s Sarcoma
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi’s sarcoma. The maximum recommended duration of treatment with EOHILIA is 12 weeks [see Dosage and Administration (2.1)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described in greater detail in other sections of the labeling:
- Hypercorticism and adrenal axis suppression [see Warnings and Precautions (5.1)]
- Immunosuppression and increased risk of infections [see Warnings and Precautions (5.2)]
- Erosive esophagitis [see Warnings and Precautions (5.3)]
- Effect on growth [see Warnings and Precautions (5.4)]
- Symptoms of steroid withdrawal in those patients transferred from other systemic corticosteroids [see Warnings and Precautions (5.5)]
- Other corticosteroid effects [see Warnings and Precautions (5.6)]
- Kaposi’s sarcoma [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of EOHILIA in 410 adults and pediatric subjects 11 years of age and older with EoE was evaluated in two 12-week, double-blind, placebo-controlled studies (Study 1 and Study 2). In these studies, 263 subjects received EOHILIA [see Clinical Studies (14)].
Common Adverse Reactions
The most common adverse reactions reported in Study 1 are shown in Table 1.
Table 1: Common Adverse Reactions* in Adult and Pediatric Subjects 11 Years of Age and Older with EoE in Study 1 ADVERSE REACTIONS EOHILIA
2 mg Twice Daily
N = 213Placebo
N = 105- *
- reported in at least 2% of subjects in the EOHILIA group and at a rate greater than placebo.
- †
- includes acute sinusitis, sinusitis, nasopharyngitis, respiratory tract infection, respiratory tract infection viral, upper respiratory tract infection, viral upper respiratory tract infection, rhinitis
- ‡
- includes esophageal candidiasis, oropharyngeal candidiasis, oral candidiasis
- §
- includes headache, migraine
- ¶
- includes throat irritation, oropharyngeal pain
- #
- includes adrenal suppression, adrenal insufficiency
- Þ
- includes esophagitis only where erosions were present at the esophagogastroduodenoscopy conducted after 12 weeks of treatment
Respiratory tract infection† 13% 11% Gastrointestinal mucosal candidiasis‡ 8% 2% Headache§ 5% 2% Gastroenteritis 3% 1% Throat irritation¶ 3% 2% Adrenal suppression# 2% 0 Erosive esophagitisÞ 2% 0 Specific Adverse Reactions
Erosive Esophagitis
Erosive esophagitis occurred in 4/213 (2%) of EOHILIA-treated subjects in Study 1. These subjects had no erosions present at the baseline esophagogastroduodenoscopy (EGD). At EGD after 12 weeks of treatment with EOHILIA, 2 subjects had Los Angeles (LA) classification grade A, 1 subject had LA grade B, and 1 subject had LA grade C erosive esophagitis. All but one of the four subjects developed erosive esophagitis while receiving concomitant therapy with a PPI. No cases of erosive esophagitis were reported in Study 2 [see Warnings and Precautions (5.3)].
Less Common Adverse Reactions
Adverse reactions reported in less than 2% of subjects treated with EOHILIA and at a greater rate than placebo in Study 1 are listed below:
Cardiac disorders: Palpitations
Gastrointestinal disorders: Dry mouth, dyspepsia, erosive duodenitis, gastrointestinal motility disorder, esophageal food impaction, palatal swelling
General disorders and administration site conditions: Fatigue, feeling abnormal
Infections and infestations: Fungal skin infection, paronychia, pneumonia, sepsis, bronchitis
Investigations: Transaminases increased, hyperkalemia
Metabolism and nutrition disorders: Dyslipidemia
Musculoskeletal and connective tissue disorders: Arthralgia, muscle spasms
Nervous system disorders: Dysgeusia, syncope, tremor
Psychiatric disorders: Depression, irritability, restlessness
Respiratory, thoracic, and mediastinal disorders: Nasal congestion
Skin and subcutaneous tissue disorders: Hirsutism, dermatitis acneiform
Vascular disorders: Hypertension
The safety profile of EOHILIA in Study 2 was generally similar to Study 1.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post-approval use of oral budesonide products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity Reactions: Anaphylaxis
Nervous System Disorders: Benign intracranial hypertension
Psychiatric Disorders: Mood swings
-
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
Budesonide is a substrate for CYP3A4. Concomitant use of budesonide with CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, erythromycin, cyclosporine, grapefruit juice) can increase systemic budesonide concentrations [see Clinical Pharmacology (12.3)]. Avoid concomitant use of CYP3A4 inhibitors, including grapefruit juice, with EOHILIA [see Dosage and Administration (2.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available data from published case series, epidemiological studies, and reviews with oral budesonide use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal outcomes. Infants exposed to in-utero corticosteroids, including EOHILIA, are at risk for hypoadrenalism (see Clinical Considerations). In animal reproduction studies, subcutaneous administration of budesonide during organogenesis in pregnant rats (at doses up to approximately 1.2 times the maximum recommended human dose, based on body surface area [BSA]) or pregnant rabbits (at doses approximately 0.14 times the maximum recommended human dose, based on body surface area [BSA]) resulted in increased fetal loss, decreased pup weights, and skeletal abnormalities. Maternal toxicity was observed in both rats and rabbits at these dose levels (see Data). Based on animal data, advise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage in the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Infants should be carefully observed for signs of hypoadrenalism, such as poor feeding, irritability, weakness, and vomiting, and managed accordingly [see Warnings and Precautions (5.1)].
Data
Animal Data
Budesonide was teratogenic and embryolethal in rabbits and rats.
In an embryo-fetal development study in pregnant rats dosed subcutaneously with budesonide during the period of organogenesis from gestation days 6-15, there were effects on fetal development and survival at subcutaneous doses up to approximately 500 mcg/kg/day in rats (approximately 1.2 times the maximum recommended human dose [MRHD], based on body surface area [BSA]). In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6-18, there was an increase in maternal abortion, and effects on fetal development and reduction in litter weights at subcutaneous doses up to approximately 25 mcg/kg/day (approximately 0.14 times the MRHD, based on BSA). Maternal toxicity, including reduction in body weight gain, was observed at subcutaneous doses of 5 mcg/kg in rabbits (approximately 0.03 times the MRHD, based on BSA) and 500 mcg/kg in rats (approximately 1.2 times the MRHD, based on BSA).
In a peri- and post-natal development study, rats dosed subcutaneously with budesonide during the period of Day 15 post coitum to Day 21 postpartum, budesonide had no effects on delivery but did have an effect on growth and development of offspring. In addition, offspring survival was reduced and surviving offspring had decreased mean body weights at birth and during lactation at exposures 0.05 times the MRHD (on a mg/m2 basis at maternal subcutaneous doses of 20 mcg/kg/day and higher). These findings occurred in the presence of maternal toxicity.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted with oral budesonide, including EOHILIA, and no information is available on the effects of the drug on the breastfed infant or the effects of the drug on milk production. One published study reported that budesonide is present in human milk following maternal inhalation of budesonide (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for EOHILIA and any potential adverse effects on the breastfed infant from EOHILIA, or from the underlying maternal condition.
Data
One published study reported that budesonide is present in human milk following maternal inhalation of budesonide which resulted in infant doses approximately 0.3% to 1% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.4 and 0.5. Budesonide plasma concentrations were not detected, and no adverse events were noted in the breastfed infants following maternal use of inhaled budesonide. The recommended daily dose of EOHILIA is higher (4 mg daily) compared with inhaled budesonide (up to 800 mcg daily) given to mothers in the above-described study.
The maximum budesonide plasma concentration following a 2 mg single dose and twice a day repeat dose of oral budesonide is approximately 0.92 ng/mL to 1.1 ng/mL which is up to 2.5 times higher than the 0.43 to 0.86 ng/mL for an 800 mcg daily dose of inhaled budesonide at steady state in the above inhalation study.
Assuming the coefficient of extrapolation between the inhaled and oral doses is constant across all dose levels, at therapeutic doses of EOHILIA, budesonide exposure to the nursing child may be up to 2.5 times higher than that by budesonide inhalation.
8.4 Pediatric Use
The safety and effectiveness of EOHILIA for 12 weeks of treatment for EoE have been established in pediatric patients 11 years of age and older. Use of EOHILIA for this indication is supported by adequate and well controlled studies in adults and pediatric subjects 11 years of age and older (Studies 1 and 2) with pharmacokinetic data in pediatric subjects aged 11 to 17 years of age. In Studies 1 and 2, the safety of EOHILIA in pediatric subjects 11 to 17 years of age was similar to the safety profile in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Systemic effects such as hypercorticism and adrenal axis suppression may occur with use of corticosteroids, including EOHILIA [see Warnings and Precautions (5.1), Adverse Reactions (6.1), and Clinical Pharmacology (12.2)].
Use of corticosteroids may cause a reduction of growth velocity in pediatric patients. Monitor the growth of pediatric patients on EOHILIA. The recommended duration of treatment with EOHILIA is 12 weeks [see Dosage and Administration (2.1)].
The safety and effectiveness of EOHILIA in pediatric patients less than 11 years of age have not been established.
Juvenile Animal Toxicity Data
In a juvenile rat study, budesonide was administered orally at doses of 0.05, 0.2, 0.6, and 1.5 mg/kg/day starting on postnatal day (PND) 22 for 91 consecutive days. All of the effects noted were associated with the pharmacology of the drug and manifested in a dose related manner, typical of corticosteroids. These included decreased body weight gain, adrenal atrophy, and effects on bone (decreased cellularity and bone length) and lymphoid tissues (decreased cellularity). The no observed adverse effect level (NOAEL) in juvenile rats was determined to be the 0.05 mg/kg/day. Plasma exposure (AUC) in rats at the NOAEL (0.05 mg/kg/day) was lower (approximately 0.8 times) than that in pediatric subjects (11 years to 17 years of age) at the MRHD.
In a juvenile toxicity study in dogs, budesonide was orally administered at doses of 0.05, 0.2, and 0.6 mg/kg/day (starting at approximately 7 weeks of age) for 91 consecutive days. The observations were dose-related and consistent with corticosteroid treatment. The effects included decreased body weight gain, distended abdomen and swelling associated with inguinal hernias, lymphocytic and leukocytic changes, adrenal atrophy, depletion in lymphoid organs (lymph nodes, spleen, thymus), and effects on skeletal development (decreased cellularity in bone marrow, bone growth, and skeletal muscle atrophy). The incidence and severity of the majority of the pharmacologically mediated effects decreased or resolved completely following the recovery period. A NOAEL could not be defined in juvenile dogs. Plasma exposure (AUC) at the lowest dose tested (0.05 mg/kg/day) was lower (approximately 0.4 times) than that in pediatric subjects (11 years to 17 years of age) at the MRHD.
8.5 Geriatric Use
Clinical studies of EOHILIA did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects. In general, EOHILIA should be used with caution due to the potential for decreased hepatic function [see Warnings and Precautions (5.1)].
8.6 Hepatic Impairment
Patients with moderate to severe hepatic impairment (Child-Pugh Class B or Class C, respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure to budesonide. Use is not recommended in patients with severe hepatic impairment (Child-Pugh Class C). The recommended dosage in patients with mild or moderate hepatic impairment (Child-Pugh Class A or Class B) is the same as the recommended dosage in patients with normal hepatic function. In patients with moderate hepatic impairment (Child-Pugh Class B), monitor for signs and/or symptoms of hypercorticism [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Treatment of acute overdosage consists of immediate gastric lavage or emesis followed by supportive and symptomatic therapy.
Use of corticosteroids, such as EOHILIA, at excessive doses or for prolonged periods, increases the risk of systemic corticosteroid effects such as hypercorticism and adrenal axis suppression [see Warnings and Precautions (5.1)].
-
11 DESCRIPTION
EOHILIA (budesonide oral suspension) contains budesonide, a synthetic corticosteroid.
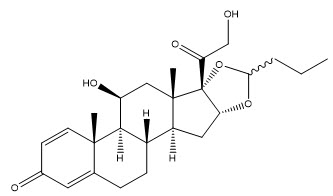
Budesonide is designated chemically as (RS)-11β, 16α, 17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide is a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.53. Its structural formula is:
Budesonide is a white or almost-white, crystalline powder that is freely soluble in chloroform, sparingly soluble in alcohol and practically insoluble in water and heptane.
EOHILIA (budesonide oral suspension) is a white to yellow, thixotropic, viscous suspension. Each 10 mL of EOHILIA contains 2 mg of budesonide. The oral suspension contains the following inactive ingredients: acesulfame potassium, ascorbic acid, Avicel® RC-591, cherry flavor, citric acid, dextrose, disodium ethylenediaminetetraacetic acid (EDTA), glycerin, Magnasweet® 110, maltodextrin, polysorbate 80, potassium sorbate, sodium ascorbate, sodium benzoate, sodium citrate, and purified water. Excipients contain no ingredient made from a gluten-containing grain (wheat, barley, or rye).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Budesonide is an anti-inflammatory corticosteroid and has a high glucocorticoid effect and a weak mineralocorticoid effect, and the affinity of budesonide to glucocorticoid receptors, which reflects the intrinsic potency of the drug, is about 200-fold that of cortisol and 15-fold that of prednisolone.
The precise mechanism of corticosteroid actions on inflammation in EoE is not known. Inflammation is an important component in the pathogenesis of EoE. Corticosteroids have a wide range of inhibitory activities against multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukocytes and cytokines) involved in allergic inflammation.
12.2 Pharmacodynamics
In Study 1, blood samples to detect cortisol levels (stimulated and unstimulated) were collected in the morning for all subjects [see Clinical Studies (14)]. At baseline, 4% of EOHILIA-treated subjects and 5% of placebo-treated subjects had abnormal peak adrenocorticotropic hormone (ACTH)-stimulated serum cortisol values, with abnormal defined as ≤18 mcg/dL. After 12 weeks of treatment, 9% of EOHILIA-treated subjects and 3% of placebo-treated subjects had abnormal peak ACTH-stimulated serum cortisol values [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
12.3 Pharmacokinetics
Budesonide geometric mean (%CV) maximum plasma concentration (Cmax) was 915 (59) pg/mL and area under the time concentration curve at 12 hours (AUC0-12h) was 5071 (58) pg•h/mL after repeated oral administration of EOHILIA 2 mg twice daily in adult subjects with EoE. Based on population pharmacokinetic analysis, there was no difference in pharmacokinetics between healthy adults and adult subjects with EoE.
Absorption
The median (range) time to reach budesonide peak plasma concentration (tmax) was 2 hours (range 0.5 to 4 hours) after oral administration of EOHILIA 2 mg twice daily in adult subjects with EoE. Following repeated (twice daily) oral administration of EOHILIA, the systemic exposure of budesonide was increased dose proportionally in the dose range of 0.5 to 2 mg (0.25 times the recommended dose and up to the recommended dose).
The oral bioavailability of budesonide in healthy subjects is estimated to be 14% under fasting state.
Effect of Food
A high-fat, high-calorie meal (800-1000 calories and approximately 50% fat) administered to healthy subjects increased the AUC of budesonide by 26% and decreased the Cmax by 13% following a single dose of EOHILIA compared to fasting conditions. Median time to reach peak plasma concentration was delayed approximately 1 hour with the intake of a high-fat, high-calorie meal. The increase in systemic exposure of budesonide due to food effect is not expected to be clinically meaningful.
Distribution
The mean volume of distribution (Vss/F) of budesonide is 1886 L after repeated oral administration of EOHILIA. Plasma protein binding was estimated to be 85% to 90% in the concentration range 0.43 to 99.02 ng/mL. The erythrocyte/plasma partition ratio at clinically relevant concentrations was about 0.8.
Elimination
Budesonide has a high plasma clearance, 0.9 to 1.8 L/min approaching the estimated liver blood flow, suggesting that budesonide is a high hepatic clearance drug. The mean plasma elimination half-life (t1/2) of budesonide after administration of EOHILIA was 3.3 hours.
Metabolism
Following oral absorption, budesonide is subject to high first pass metabolism (80% to 90%). In vitro, budesonide is biotransformed, mainly by CYP3A4, to its 2 major metabolites, 6beta-hydroxy budesonide and 16alpha-hydroxy prednisolone. The corticosteroid activity of these metabolites was negligible (less than1/100) in relation to that of the parent compound.
Excretion
Budesonide is excreted in urine and feces in the form of metabolites. After oral as well as intravenous administration of micronized [3H]-budesonide, approximately 60% of the recovered radioactivity was found in urine. The major metabolites, including 6beta-hydroxy budesonide and 16alpha-hydroxy prednisolone, are mainly renally excreted, intact or in conjugated forms. No unchanged budesonide was detected in urine.
Specific Populations
Pediatric Patients
The pharmacokinetics of budesonide were evaluated in pediatric subjects aged 11 years to 17 years (n=10) following oral administration of EOHILIA 2 mg twice a day. The median (range) time to peak plasma concentration of budesonide was 1 (0.5, 2) hour, the mean (%CV) Cmax was 946 (61%) pg/mL and the mean (%CV) AUC0-8h was 3849 (51%) pg•h/mL.
Based on a population pharmacokinetic analysis, the steady-state exposure following EOHILIA 2 mg twice daily is predicted to be comparable between pediatric subjects (11 to less than 18 years) and adults (18 years and older).
Patients with Hepatic Impairment
In subjects with mild (Child-Pugh Class A, n=4) or moderate (Child-Pugh Class B, n=4) hepatic impairment, budesonide 4 mg was administered orally as a single dose. The subjects with moderate hepatic impairment had a 3.5-fold higher AUC compared to the healthy subjects with normal hepatic function while the subjects with mild hepatic impairment had an approximately 1.4-fold higher AUC. The Cmax values demonstrated similar increases [see Warnings and Precautions (5.1)]. The increased systemic exposure in subjects with mild hepatic impairment was not considered to be clinically relevant. Subjects with severe hepatic impairment (Child-Pugh Class C) were not studied [see Use in Specific Populations (8.6)].
Drug Interaction Studies
Budesonide is a substrate of CYP3A4. Inhibitors of CYP3A4 can increase the plasma concentrations of budesonide several-fold. Conversely, induction of CYP3A4 potentially could result in the lowering of budesonide plasma concentrations [see Dosage and Administration (2.2) and Drug Interactions (7.1)].
Budesonide is a substrate of P-gp, but not a substrate of BCRP, OATP1B1, OATP1B3, OAT1, OAT3, or OCT2. The role of P-gp on budesonide disposition is anticipated to be minimal due to CYP3A-mediated clearance.
Effects of Other Drugs on Budesonide
Ketoconazole
In an open, non-randomized, cross-over study, 6 healthy subjects were given budesonide 10 mg as a single dose (5-times the recommended dose of EOHILIA), either alone or concomitantly with the last ketoconazole dose of 3 days treatment with ketoconazole 100 mg twice daily. Coadministration of ketoconazole resulted in an eight-fold increase in AUC of budesonide, compared to budesonide alone [see Drug Interactions (7.1)].
Grapefruit Juice
In an open, randomized, cross-over study, 8 healthy subjects were given budesonide delayed-release capsules 3 mg (1.5-times the recommended dose of EOHILIA), either alone, or concomitantly with 600 mL concentrated grapefruit juice (which inhibits CYP3A4 activity predominantly in the intestinal mucosa), on the last of 4 daily administrations. Concomitant administration of grapefruit juice resulted in a 2-fold increase of the bioavailability of budesonide compared to budesonide alone [see Drug Interactions (7.1)].
Oral Contraceptives (CYP3A4 Substrates)
In a parallel study, the pharmacokinetics of budesonide were not significantly different between healthy female subjects who received oral contraceptives containing desogestrel 0.15 mg and ethinyl estradiol 30 mcg and healthy female subjects who did not receive oral contraceptives. Budesonide 4.5 mg (2.3-times the recommended dose of EOHILIA) once daily for one week did not affect the plasma concentrations of ethinyl estradiol, a CYP3A4 substrate.
Omeprazole
In a study in 11 healthy subjects, performed in a double-blind, randomized, placebo-controlled manner, the effect of 5 to 6 days treatment with omeprazole 20 mg once daily on the pharmacokinetics of budesonide administered as budesonide delayed-release capsules 9 mg (4.5-times the recommended dose of EOHILIA) as a single dose was investigated. Omeprazole 20 mg once daily did not affect the absorption or pharmacokinetics of budesonide.
Cimetidine
In an open, non-randomized, cross-over study, the potential effect of cimetidine on the pharmacokinetics of budesonide was studied. Six healthy subjects received cimetidine 1 gram daily (200 mg with meals and 400 mg at night) for 2 separate 3-day periods. Budesonide 4 mg (2-times the recommended dose of EOHILIA) was administered either alone or on the last day of one of the cimetidine treatment periods. Co-administration of cimetidine resulted in a 52% and 31% increase in the budesonide peak plasma concentration and the AUC of budesonide, respectively.
Effect of Budesonide on Other Drugs
Budesonide is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6 in vitro. Budesonide is not an inducer of CYP3A4, CYP1A2, or CYP2B6 at clinically relevant concentrations.
In vitro, budesonide is not a significant inhibitor of P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, or OCT2 at clinically relevant concentrations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Carcinogenicity studies with budesonide were conducted in rats and mice. In a 2-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in male rats at an oral dose of 50 mcg/kg/day (approximately 0.1 times the maximum recommended human dose [MRHD], based on the body surface area [BSA]). In addition, there were increased incidences of primary hepatocellular tumors in male rats at 25 mcg/kg (approximately 0.06 times the MRHD, based on BSA) and above. No tumorigenicity was seen in female rats at oral doses up to 50 mcg/kg/day (approximately 0.1 times the MRHD, based on BSA).
In an additional 2-year study in male Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg/day (approximately 0.1 times the MRHD, based on BSA); however, it caused a statistically significant increase in the incidence of hepatocellular tumors at this same oral dose of 50 mcg/kg/day. The concurrent reference corticosteroids (prednisolone and triamcinolone acetonide) showed similar findings. In a 91-week study in mice, budesonide caused no treatment-related carcinogenicity at oral doses up to 200 mcg/kg/day (approximately 0.2 times the MRHD, based on BSA).
Mutagenesis
Budesonide showed no evidence of genotoxic potential in the Ames test, the mouse lymphoma cell forward gene mutation (TK+/-) test, the human lymphocyte chromosome aberration test, the Drosophila melanogaster sex-linked recessive lethality test, the rat hepatocyte UDS test or the mouse micronucleus test.
Impairment of Fertility
In rats, budesonide had no effect on fertility at subcutaneous doses up to 80 mcg/kg/day (approximately 0.1 times the MRHD, based on BSA). However, it caused a decrease in prenatal viability and viability in pups at birth and during lactation, along with a decrease in maternal body weight gain, at subcutaneous doses of 20 mcg/kg/day (approximately 0.04 times the MRHD, based on BSA) and above. No such effects were noted at 5 mcg/kg/day (approximately 0.01 times the MRHD on a body surface area basis).
-
14 CLINICAL STUDIES
The efficacy and safety of EOHILIA 2 mg twice daily were evaluated in two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies (Study 1 [NCT02605837] and Study 2 [NCT01642212]). Eligible subjects in Study 1 and Study 2 had esophageal inflammation defined as ≥15 eosinophils/high-power field (hpf) from at least 2 levels of the esophagus at baseline following a treatment course of a proton pump inhibitor (PPI) either prior to or during screening and at least 4 days of dysphagia as measured by the Dysphagia Symptom Questionnaire (DSQ) over a 2-week period prior to randomization. Concomitant use of stable doses of inhaled or intranasal steroids (for conditions other than EoE), PPIs, H2-receptor antagonists, antacids, antihistamines or anti-leukotrienes, and maintenance immunotherapy was allowed. In Study 1, subjects were enrolled after maintaining a stable diet for at least 3 months prior to screening and were instructed to maintain a stable diet throughout the study. Subjects were excluded if they were on a full liquid or 6-food elimination diet. In Study 2, subjects were instructed to maintain a stable diet throughout the study. In Study 1 and Study 2, subjects were instructed to not eat or drink for 30 minutes after taking the drug and then to rinse their mouth with water and spit out the contents without swallowing prior to resuming normal oral intake.
A total of 318 subjects (277 adults and 41 pediatric subjects) were randomized and received at least one dose of study drug (EOHILIA or placebo) in Study 1. The mean age of the study population was 34 years (range 11 to 56 years). Sixty percent of subjects were male, 95% were White, and 3% were Hispanic or Latino. Over 80% of the subjects were on concomitant PPI. The mean (SD) DSQ combined scores at baseline were 30.3 (13.9) and 30.4 (13.1) in the EOHILIA and placebo groups, respectively.
A total of 92 subjects (58 adults and 34 pediatric subjects) were randomized and received at least one dose of study drug (EOHILIA or placebo) in Study 2. The mean age of the study population was 22 years (range 11 to 42 years). Sixty-eight percent of subjects were male, 95% were White, and 1% were Hispanic or Latino. Over 65% of the subjects were on concomitant PPI. The mean (SD) DSQ combined scores at baseline were 30.7 (16.0) and 29.0 (13.5) in the EOHILIA and placebo groups, respectively.
Study 1 and Study 2 evaluated efficacy endpoints of histologic remission (defined as a peak eosinophil count of ≤6/hpf across all available esophageal levels) and the absolute change from baseline in subject-reported DSQ combined score after 12 weeks of treatment.
Efficacy results for Study 1 and Study 2 are presented in Table 2.
Table 2: Efficacy Results of EOHILIA in Adult and Pediatric Subjects 11 Years of Age and Older with EoE after 12 Weeks (Study 1 and Study 2) Study 1 Study 2 EOHILIA
2 mg Twice Daily
N=213Placebo
N=105Treatment difference and 95% CI* EOHILIA
2 mg Twice Daily
N=50Placebo
N=42Treatment difference and 95%CI* - *
- For histological remission, the difference in percentages and 95% Newcombe confidence intervals are estimated using Mantel Haenszel weights, adjusting for age group and diet restriction. For absolute change in DSQ score, the LS mean changes, standard errors, and differences are estimated using an ANCOVA model with treatment group, age group, diet restriction, and baseline measurement as covariates.
- †
- Total biweekly DSQ scores range from 0 to 84, higher scores indicate greater frequency and severity of dysphagia
Efficacy Endpoints Proportion of subjects achieving histological remission (peak esophageal intraepithelial eosinophil count ≤6 eos/hpf) 53.1% 1.0% 52.4%
(43.3, 59.1)38.0% 2.4% 35.8%
(17.2, 50.0)Absolute change from baseline in DSQ combined score (0-84†), LS mean (SE) -10.2 (1.5) -6.5 (1.8) -3.7
(-6.8, -0.6)-14.5 (1.8) -5.9 (2.1) -8.6
(-13.7, -3.5)During the last 2 weeks of the 12-week treatment periods in Study 1 and Study 2, a greater proportion of subjects randomized to EOHILIA experienced no dysphagia or only experienced dysphagia that “got better or cleared up on its own” compared to placebo, as measured by the subject-reported DSQ.
Additional Study
After completing Study 1, 48 subjects from the EOHILIA 2 mg treatment arm entered a double-blind randomized withdrawal extension study. These subjects received EOHILIA 2 mg twice daily or placebo for up to an additional 36 weeks. Treatment with EOHILIA did not demonstrate a statistically significant difference compared to subjects re-randomized to placebo for prespecified efficacy endpoints based on eosinophil count and/or clinical symptoms measured by the DSQ at Week 36.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
- EOHILIA (budesonide oral suspension) 2 mg/10 mL is a white to yellow viscous suspension with cherry flavoring supplied in child-resistant unit-dose packets.
- EOHILIA is supplied in a carton containing 60 unit-dose packets (NDC 64764-105-60).
Store refrigerated or at controlled room temperature at 2°C to 25°C (36°F to 77°F). Excursions up to 30°C (86°F) are acceptable. Do NOT freeze.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Hypercorticism and Adrenal Axis Suppression
Advise the patient or caregiver that EOHILIA may cause hypercorticism and adrenal axis suppression and to report any signs or symptoms to their healthcare provider (e.g., acne, bruise easily, ankle swelling, thicker body hair and facial hair, pink or purple stretch marks, a fatty pad or hump between the shoulders, tiredness, weakness, nausea and vomiting, low blood pressure) [see Warnings and Precautions (5.1)].
Immunosuppression and Increased Risk of Infection
Advise patients or caregivers to inform their healthcare provider if they develop a new or worsening infection and to contact their healthcare provider immediately if they are exposed to varicella or measles [see Warnings and Precautions (5.2)].
Inform the patient or caregiver that localized infections with Candida albicans may occur in the mouth, throat, and esophagus. Instruct patients to rinse the mouth with water 30 minutes after administration of EOHILIA and to spit out the contents without swallowing. Advise the patient or caregiver to contact the healthcare provider if the patient experiences signs or symptoms of oropharyngeal or esophageal candidiasis [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
Erosive Esophagitis
Advise patients or caregivers to report new onset or worsening signs or symptoms of erosive esophagitis (e.g., heartburn, chest pain, trouble swallowing) to their healthcare provider [see Warnings and Precautions (5.3)].
Effect on Growth
Advise patients or caregivers that corticosteroids can affect growth in pediatric patients and to report concerns regarding growth while taking EOHILIA to the healthcare provider [see Warnings and Precautions (5.4)].
Symptoms of Steroid Withdrawal in Patients Transferred from Other Systemic Corticosteroids
If transferring to EOHILIA from corticosteroid treatment with high systemic effects, advise the patient or caregiver to follow a taper schedule for systemic corticosteroids, as instructed by the healthcare provider. Advise patients or caregivers that replacement of other systemic corticosteroids with EOHILIA may unmask allergies (e.g., rhinitis and eczema), which were previously controlled by the other drug [see Warnings and Precautions (5.5)].
Kaposi’s Sarcoma
Advise patients or caregivers that Kaposi’s sarcoma has been reported in patients receiving corticosteroids for chronic conditions and to inform their healthcare provider if they experience signs or symptoms of Kaposi’s sarcoma [see Warnings and Precautions (5.7)].
Pregnancy
Advise female patients that EOHILIA may cause fetal harm and to inform their healthcare provider with a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Administration
Advise patients:
- Do NOT take EOHILIA with food or liquid at the time of ingestion. Wait for at least 30 minutes to eat or drink after taking EOHILIA [see Clinical Pharmacology (12.3)].
- Administer EOHILIA as follows:
- Do NOT mix EOHILIA with food or liquid.
- Shake EOHILIA packet for at least 10 seconds prior to opening.
- Squeeze the packet from the bottom to the top directly into the mouth. Repeat 2 to 3 times until the EOHILIA packet is empty.
- Swallow all the EOHILIA suspension.
- Do not eat or drink for 30 minutes after taking EOHILIA. After 30 minutes, rinse mouth with water and spit out the contents without swallowing [see Dosage and Administration (2.2), Warnings and Precautions (5.2)].
- Avoid consumption of grapefruit juice for the duration of EOHILIA therapy [see Drug Interactions (7.1)].
Distributed by:
Takeda Pharmaceuticals America, Inc.
Cambridge, MA 02142
EOHILIA and
 are trademarks of ViroPharma Biologics LLC.
are trademarks of ViroPharma Biologics LLC.TAKEDA and
 are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.©2025 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
EOH357 R4
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 1/2025 EOH357 R4 PATIENT INFORMATION
EOHILIA™ (ee-oh-HIL-ee-uh)
(budesonide oral suspension)What is EOHILIA?
EOHILIA is a prescription oral corticosteroid medicine used for 12 weeks of treatment of Eosinophilic Esophagitis (EoE), in people 11 years and older.
EOHILIA has not been shown to be safe and effective for the treatment of EoE for longer than 12 weeks.
It is not known if EOHILIA is safe and effective in children younger than 11 years of age.
Who should not take EOHILIA?
Do not take EOHILIA if:- you are allergic to budesonide.
Before you take EOHILIA tell your healthcare provider if you have any other medical conditions including if you: - have liver problems.
- are planning to have surgery.
- have chicken pox or measles or have recently been near anyone with chicken pox or measles.
- have certain kinds of infection that have not been treated including:
- fungal infections.
- bacterial infections.
- viral infections.
- parasitic infections, including threadworm (Strongyloides) infections.
- herpes simplex infection of the eye (ocular herpes simplex).
- have or had tuberculosis.
- have malaria of the brain (cerebral malaria).
- have an infection of the mouth, throat, or esophagus.
- have diabetes or glaucoma or have a family history of diabetes or glaucoma.
- have cataracts.
- have high blood pressure (hypertension).
- have low bone mineral density or osteoporosis.
- have stomach ulcers.
- are pregnant or plan to become pregnant. EOHILIA may harm your unborn baby. Talk to your healthcare provider about the possible risk to your unborn baby if you take EOHILIA when you are pregnant. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during your treatment with EOHILIA.
- are breastfeeding or plan to breastfeed. It is not known if EOHILIA passes into your breast milk or if it will affect your baby. Talk to your healthcare provider about the best way to feed your baby if you take EOHILIA.
How should I take EOHILIA? - See the detailed Instructions for Use that comes with EOHILIA for information about how to prepare and take EOHILIA and how to properly store and throw away (dispose of) used EOHILIA packets.
- Take EOHILIA exactly as your healthcare provider tells you. Your healthcare provider will tell you how much EOHILIA to take.
- EOHILIA is taken by mouth, 2 times a day (1 time in the morning and 1 time in the evening).
- Do not mix EOHILIA with food or liquid.
- Do not eat or drink at the same time as taking EOHILIA. Wait to eat or drink at least 30 minutes after taking EOHILIA.
- Your healthcare provider may change your dose if needed. Do not change your dose or stop taking EOHILIA unless your healthcare provider tells you.
- Avoid drinking grapefruit juice while taking EOHILIA. Drinking grapefruit juice can increase the level of EOHILIA in your blood.
What are the possible side effects of EOHILIA?
EOHILIA may cause serious side effects, including:- Effects of having too much corticosteroid medicine in your blood (hypercorticism). Long-term use of EOHILIA may cause you to have elevated levels of corticosteroid medicine in your blood. Tell your healthcare provider if you have any of the following signs and symptoms:
- acne
- bruise easily
- rounding of your face
- ankle swelling
- thicker body hair and facial hair
- a fatty pad or hump between your shoulders (buffalo hump)
- pink or purple stretch marks on the skin of your abdomen, thighs, breasts and arms
- Adrenal suppression. Long-term use of EOHILIA can cause a condition in which the adrenal glands do not make enough steroid hormones (adrenal suppression). Tell your healthcare provider if you are under stress or if you have any of the following signs or symptoms:
- tiredness
- weakness
- nausea and vomiting
- low blood pressure
-
Decreased ability of your body to fight infections (immunosuppression) and increased risk of infection. Corticosteroid medicines, including EOHILIA, lower the ability of your immune system to fight infections and increase the risk of infections caused by viruses, bacteria, fungi, protozoans, or certain parasites. Corticosteroid medicines, including EOHILIA can also:
- make current infections worse
- increase the risk of infections spreading (disseminated)
- increase the risk of making infections active again or making infections worse that have not been active (latent)
- hide (mask) some signs of infection
- fever
- chills
- stomach area (abdominal) pain
- aches
- diarrhea
- cough
- pain
- feeling tired
- nausea and vomiting
- Tuberculosis: If you have inactive (latent) tuberculosis, your tuberculosis may become active again while taking EOHILIA. Your healthcare provider should check you closely for signs and symptoms of tuberculosis while taking EOHILIA.
- Chicken pox and measles: People taking corticosteroid medicines, including EOHILIA, who have not had chicken pox or measles, should avoid contact with people who have these diseases. Tell your healthcare provider right away if you come in contact with anyone who has chicken pox or measles.
- Hepatitis B virus (HBV) reactivation: If you are a carrier of HBV, the virus can become an active infection again while taking EOHILIA. Your healthcare provider will test you for HBV before you start taking EOHILIA.
- Amebiasis: Inactive (latent) amebiasis may become an active infection while taking EOHILIA. Your healthcare provider should check you for amebiasis before you start taking EOHILIA if you have spent time in the tropics or have unexplained diarrhea.
- Fungal infections of the mouth (thrush), throat, and esophagus in patients using EOHILIA may occur. Symptoms of infection include white spots in the mouth, a burning or painful sensation in your mouth, redness inside of your mouth, difficulty with eating or swallowing, loss of taste, and cotton feeling in your mouth. Tell your healthcare provider if any of the above symptoms occur.
- Erosive esophagitis. EOHILIA can cause acid-related damage to the lining of the esophagus. Tell your healthcare provider if you notice any new or worsening signs or symptoms:
- heartburn
- chest pain
- trouble swallowing
- Effect on growth. Taking corticosteroids can affect your child’s growth. Tell your healthcare provider if you are worried about your child’s growth. Your healthcare provider may monitor the growth of your child while taking EOHILIA.
- Worsening of allergies. If you take certain other corticosteroid medicines to treat allergies, switching to EOHILIA may cause your allergies to come back. These allergies may include a skin condition called eczema or inflammation inside your nose (rhinitis). Tell your healthcare provider if any of your allergies become worse while taking EOHILIA.
- Kaposi’s sarcoma: Kaposi’s sarcoma has happened in people who receive corticosteroid therapy, most often for treatment of long-lasting (chronic) conditions.
The most common side effects of EOHILIA include: - respiratory tract infection
- fungal infections of the mouth, throat and esophagus (thrush)
- headache
- infection of the stomach and intestine (gastroenteritis)
- sore throat
- adrenal suppression
- acid-related damage to the lining of the esophagus (erosive esophagitis)
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of EOHILIA. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store EOHILIA?
Store between 36°F to 77°F (2°C to 25°C). May be refrigerated. Do not freeze.
Keep EOHILIA and all medicines out of the reach of children.General information about the safe and effective use of EOHILIA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EOHILIA for a condition for which it was not prescribed.
Do not give EOHILIA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about EOHILIA that is written for health professionals.What are the ingredients in EOHILIA?
Active ingredient: budesonide
Inactive ingredients: acesulfame potassium, ascorbic acid, Avicel® RC‑591, cherry flavor, citric acid, dextrose, disodium ethylenediaminetetraacetic acid (EDTA), glycerin, Magnasweet® 110, maltodextrin, polysorbate 80, potassium sorbate, sodium ascorbate, sodium benzoate, sodium citrate, and purified water. Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye).
Distributed by: Takeda Pharmaceuticals America, Inc., Cambridge, MA 02142
EOHILIA and are trademarks of ViroPharma Biologics LLC. TAKEDA and
are trademarks of ViroPharma Biologics LLC. TAKEDA and  are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.
©2025 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
For more information, go to www.EOHILIA.com or call 1-877-TAKEDA-7 (1-877-825-3327). -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
EOHILIA™ (ee-oh-HIL-ee-uh)
(budesonide oral suspension)This Instructions for Use contains information on how to take EOHILIA.
Important Information You Need to Know Before Taking EOHILIA
For oral use only (take by mouth).
Take EOHILIA exactly as your healthcare provider tells you. Your healthcare provider will tell you how much EOHILIA to take.
- EOHILIA is taken by mouth, 2 times a day (1 time in the morning and 1 time in the evening).
- Do not mix EOHILIA with food or liquid.
- Avoid drinking grapefruit juice while taking EOHILIA.
- Do not eat or drink at the same time as taking EOHILIA. Wait to eat or drink at least 30 minutes after taking EOHILIA.
- Your healthcare provider may change your dose if needed. Do not change your dose or stop taking EOHILIA unless your healthcare provider tells you.
EOHILIA is provided as a unit-dose 10 mL packet intended for oral use.
Inspect package and do not take if:
- carton seal is broken or
- the packet is damaged or leaking.
- the expiration date (EXP) has passed.
Taking EOHILIA
How to take EOHILIA PACKET:
Step 1 - Do not take EOHILIA with food or liquid (see Figure A).
Step 2 - Shake the packet well for at least 10 seconds before opening (see Figure B).
- Using scissors, cut along the dotted line straight across the top of the packet.
Step 3 - Take EOHILIA by squeezing the packet from the bottom to the top directly into the mouth (see Figure C).
- Repeat 2 to 3 times until all the medicine is given.
- Swallow all the EOHILIA suspension.
- Throw away (discard) the empty packet in the household trash.
Step 4
Figure D- Do not eat or drink for 30 minutes after taking EOHILIA (see Figure D).
- After 30 minutes, rinse your mouth with water and spit out the contents without swallowing.
Storing EOHILIA
Store between 36°F to 77°F (2°C to 25°C). May be refrigerated.
Do not freeze.
Keep EOHILIA and all medicines out of the reach of children.
Disposing of EOHILIA
Throw away (discard) the empty EOHILIA packet in the household trash.
For more information, go to www.EOHILIA.com or call 1-877-TAKEDA-7 (1-877-825-3327).
Distributed by:
Takeda Pharmaceuticals America, Inc.
Cambridge, MA 02142EOHILIA and
 are trademarks of ViroPharma Biologics LLC.
are trademarks of ViroPharma Biologics LLC.TAKEDA and
 are registered trademarks of Takeda Pharmaceutical Company Limited.
are registered trademarks of Takeda Pharmaceutical Company Limited.©2025 Takeda Pharmaceuticals U.S.A., Inc. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 1/2025
EOH357 R4
- PRINCIPAL DISPLAY PANEL - 10 mL packets Carton
-
INGREDIENTS AND APPEARANCE
EOHILIA
budesonide suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64764-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Budesonide (UNII: Q3OKS62Q6X) (Budesonide - UNII:Q3OKS62Q6X) Budesonide 2 mg in 10 mL Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Maltodextrin (UNII: 7CVR7L4A2D) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) EDETATE DISODIUM (UNII: 7FLD91C86K) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Polysorbate 80 (UNII: 6OZP39ZG8H) Glycerin (UNII: PDC6A3C0OX) Sodium Ascorbate (UNII: S033EH8359) Ascorbic Acid (UNII: PQ6CK8PD0R) Sodium Benzoate (UNII: OJ245FE5EU) Potassium Sorbate (UNII: 1VPU26JZZ4) Acesulfame Potassium (UNII: 23OV73Q5G9) WATER (UNII: 059QF0KO0R) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) CHERRY (UNII: BUC5I9595W) Product Characteristics Color WHITE (White to Yellow Suspension) Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64764-105-60 60 in 1 CARTON 02/09/2024 1 NDC:64764-105-10 10 mL in 1 POUCH; Type 0: Not a Combination Product 2 NDC:64764-105-00 10 in 1 CARTON 02/09/2024 2 NDC:64764-105-01 10 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213976 02/09/2024 Labeler - TAKEDA PHARMACEUTICALS AMERICA, INC. (039997266)