Label: BLUE STAR MEDICATED- camphor ointment
- NDC Code(s): 71687-2010-2
- Packager: Focus Consumer Healthcare, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
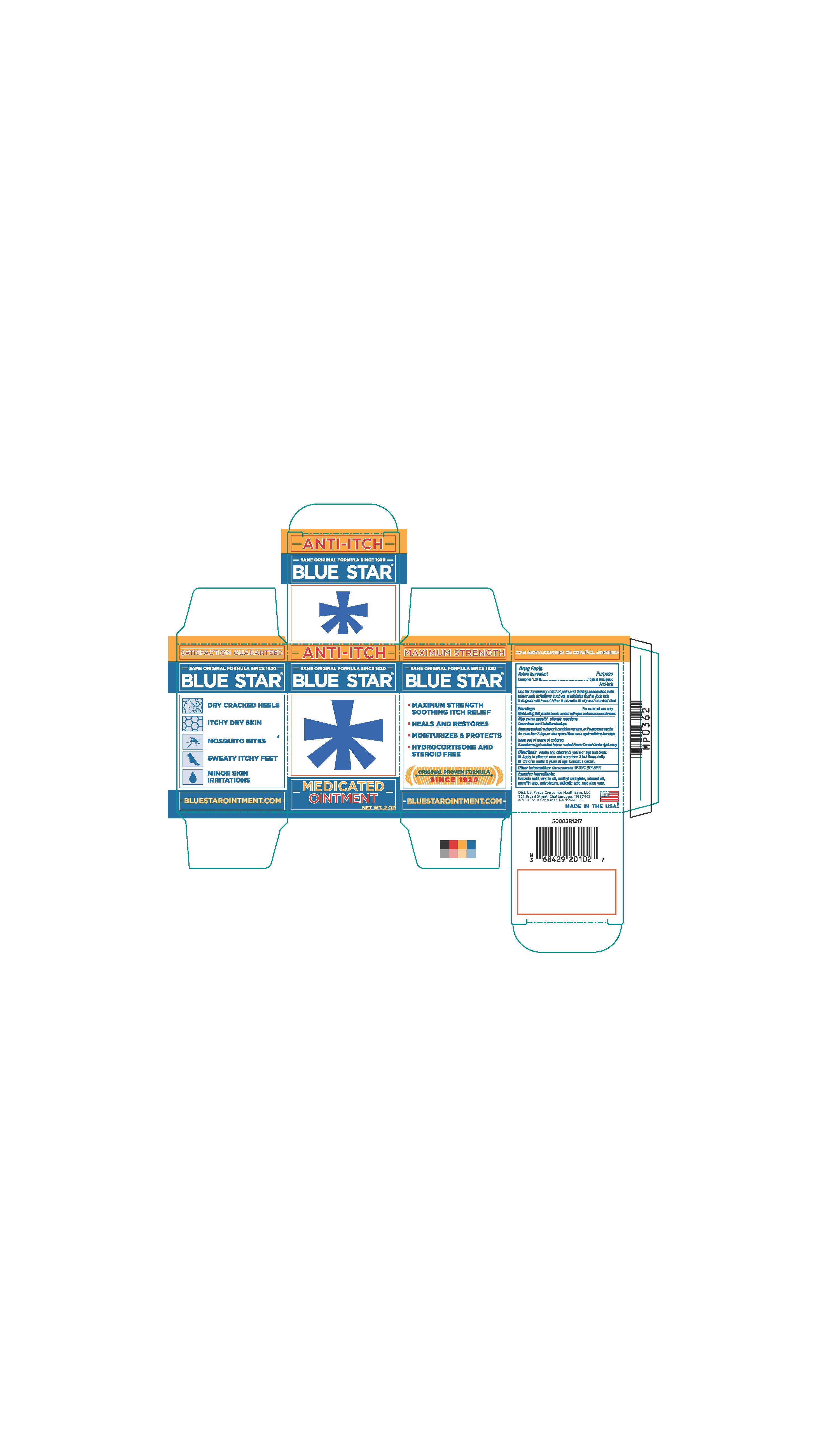
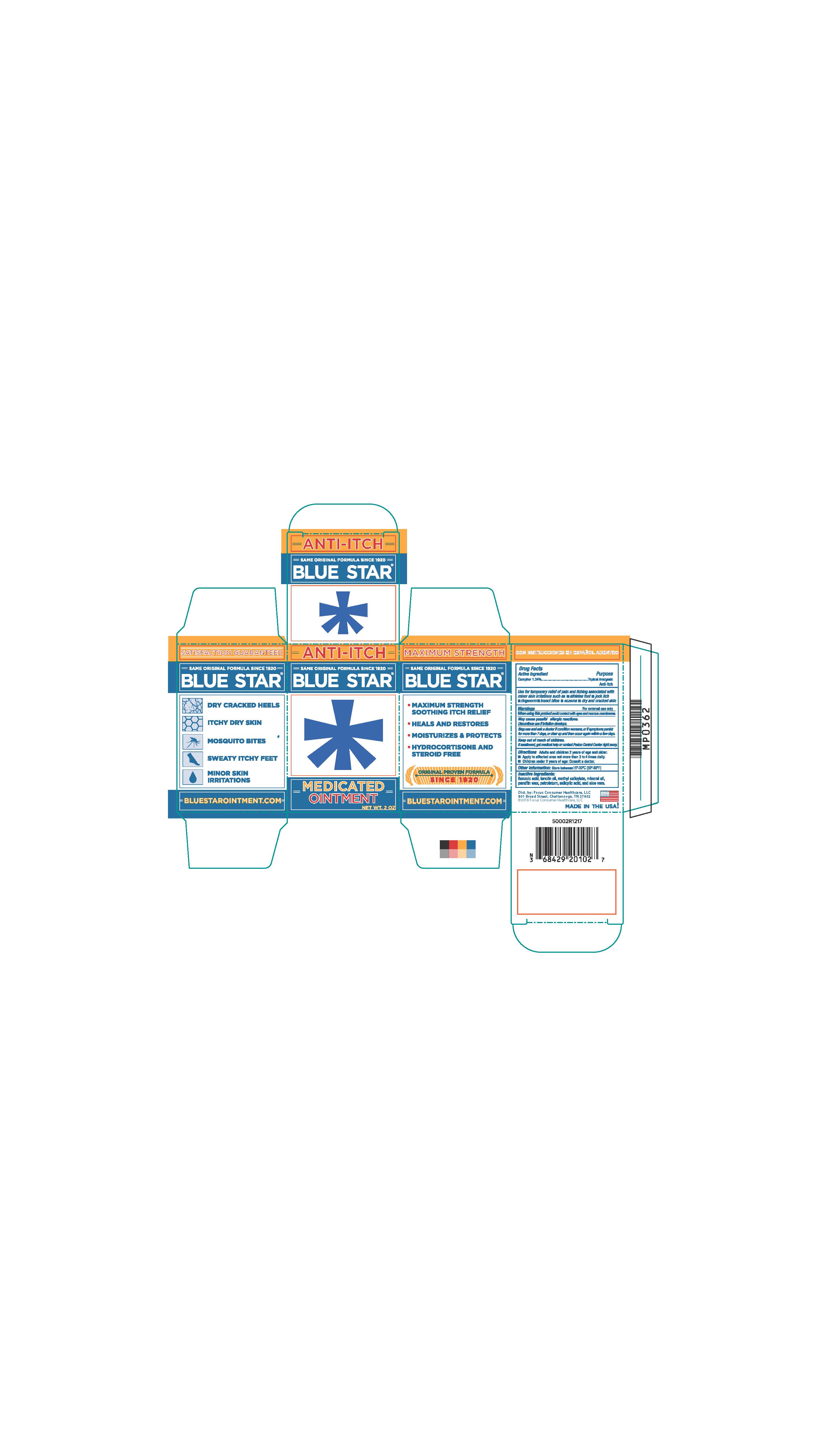
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BLUE STAR MEDICATED
camphor ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71687-2010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1.24 g in 100 g Inactive Ingredients Ingredient Name Strength LANOLIN OIL (UNII: OVV5IIJ58F) METHYL SALICYLATE (UNII: LAV5U5022Y) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) SALICYLIC ACID (UNII: O414PZ4LPZ) PARAFFIN (UNII: I9O0E3H2ZE) ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZOIC ACID (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71687-2010-2 1 in 1 CARTON 12/11/2017 1 56 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/13/2017 Labeler - Focus Consumer Healthcare, LLC (080743737)