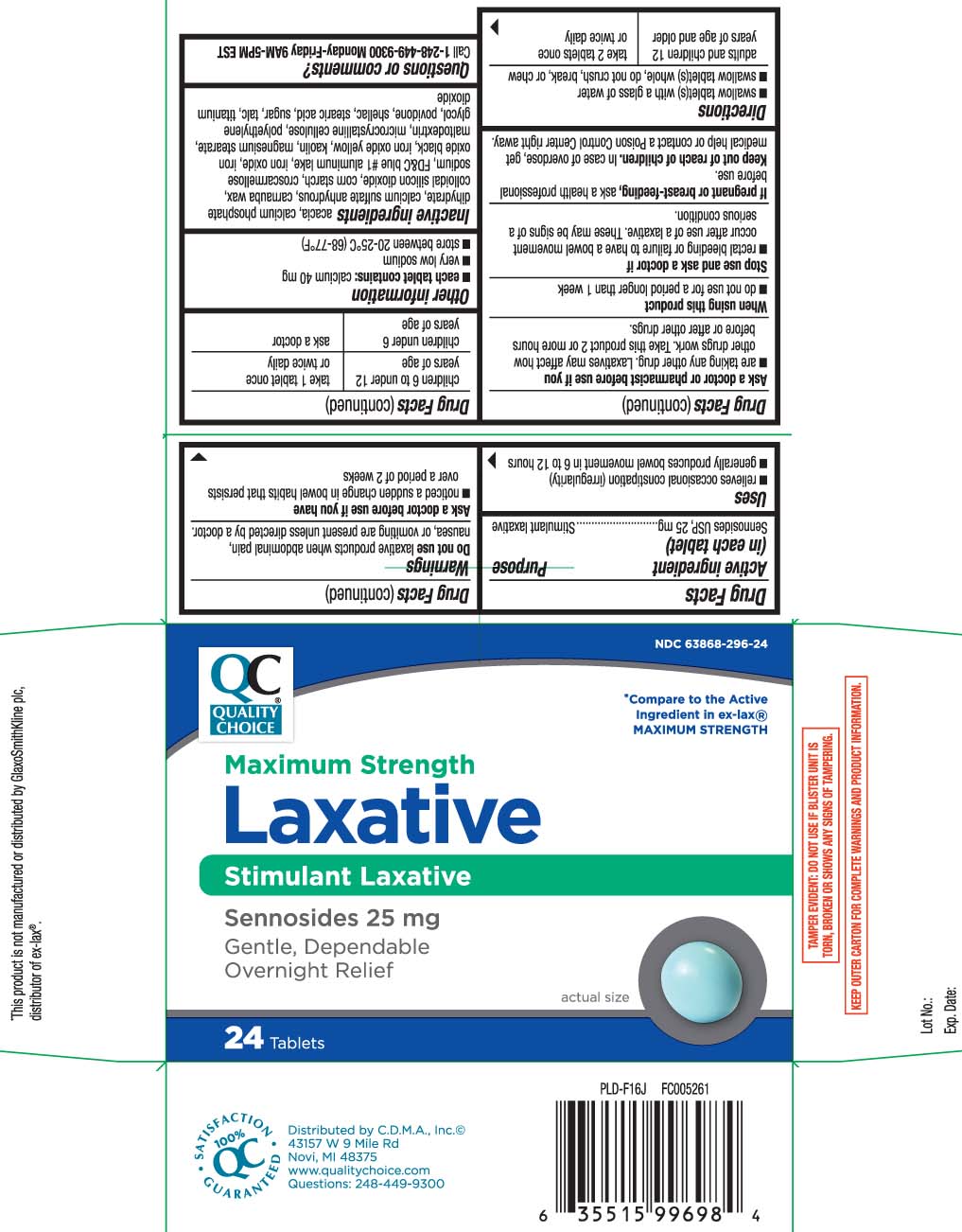
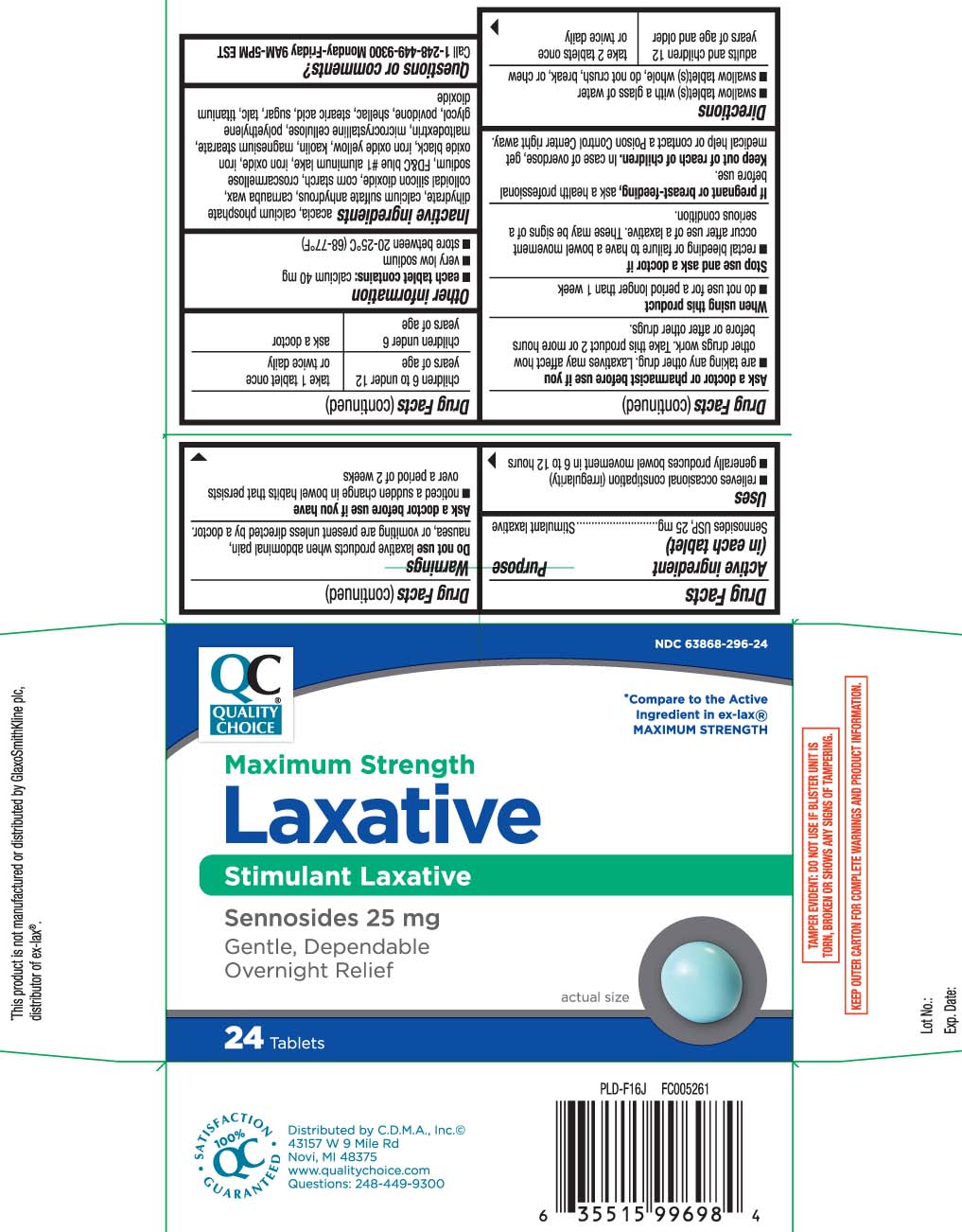
Label: LAXATIVE PILLS MAXIMUM STRENGTH- sennosides tablet
- NDC Code(s): 63868-296-24
- Packager: QUALITY CHOICE (Chain Drug Marketing Association)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
Ask a doctor before use if you have
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
Ask a doctor or pharmacist before use if you
- are taking any other drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after other drugs.
- Directions
- Other information
-
Inactive ingredients
acacia, calcium phosphate dihydrate, calcium sulfate anhydrous, carnauba wax, colloidal silicon dioxide, corn starch, croscarmellose sodium, FD&C blue #1 aluminum lake, iron oxide*, iron oxide black, iron oxide yellow, kaolin, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, shellac, stearic acid*, sugar,, talc, titanium dioxide
- Questions or comments?
-
Principal Display Panel
*Compare to the active ingredient in ex-lax® MAXIMUM STRENGTH
Maximum Strength
Laxative
Stimulant Laxative
Sennosides USP, 25 mg
Gentle, Dependable Overnight Relief
Tablets
*This product is not manufactured or distributed by GlaxoSmithKline Ex-Lax®.plc, distributor of ex-lax®.
TAMPER EVIDENT: DO NOT USE IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by C.D.M.A., Inc.©
43157 W 9 Mile Rd
Novi, MI 48375
- Product Label
-
INGREDIENTS AND APPEARANCE
LAXATIVE PILLS MAXIMUM STRENGTH
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-296 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 25 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SHELLAC (UNII: 46N107B71O) STEARIC ACID (UNII: 4ELV7Z65AP) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MALTODEXTRIN (UNII: 7CVR7L4A2D) Product Characteristics Color blue Score no score Shape ROUND Size 11mm Flavor Imprint Code TCL083 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-296-24 24 in 1 CARTON 08/31/2018 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 08/31/2018 Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774)